Tisotumab Vedotin: Synthesis and Introduction
Synthesis of Tisotumab Vedotin
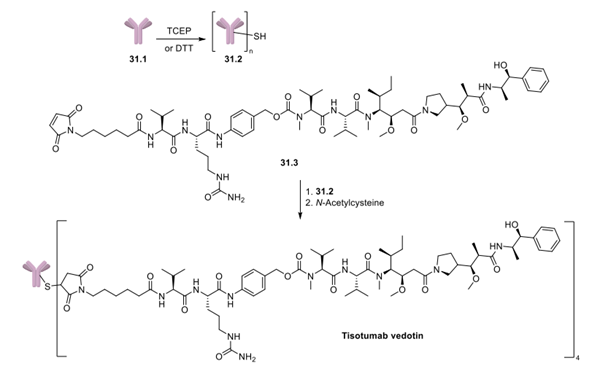
Tisotumab Vedotin may be obtained by conjugating Vedotin to a TF monoclonal antibody. The specific synthesis steps are as follows:
Tisotumab vedotin exists in a 4:1 ratio of linker-payload to antibody and is likely synthesized in a similar manner to other ADCs that use vedotin (Adcetris, Polivy, and Padcev), in which conjugation of the maleimide linker to the TF monoclonal antibody (31.1) is effected by TCEP- or DTT-mediated reduction of disulfide bonds of the antibody to reveal sulfhydryl groups in 31.2. Tisotumab vedotin 31 is then generated by subsequent reaction of the sulfhydryl groups in 31.2 with the maleimide linker functionality in 31.3, followed by quenching of any unreacted linker-payload with excess N-acetylcysteine.
Introduction of Tisotumab Vedotin
Tisotumab vedotin is an ADC that received accelerated approval in the US for patients with recurring or metastatic cervical cancer that progressed during or after chemotherapy. It is the first ADC approved that targets tissue factor (TF) as the cell-surface receptor, and it carries a black box warning of ocular toxicity. It is being co-developed by Genmab A/S and Seagen Inc., and there are other ongoing clinical trials evaluating tisotumab vedotin for other solid tumors.
Tisotumab vedotin is composed of a human monoclonal antibody for TF conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE). It utilizes the known protease-cleavable maleimidocaproyl valine-citrulline paraaminobenzoyloxycarbonyl (mc-vc-PABC) linker construct used in other known ADC drugs. TF, also known as thromboplastin, factor III, or CD142, is involved in coagulation, cell adhesion, cell motility, and angiogenesis. It is expressed in a number of tumor types and is associated with poor disease prognosis. TF has been shown to have a high internalization rate and high turnover in vitro, which makes it an attractive receptor for ADC development. MMAE is a potent and toxic antiproliferative that blocks microtublin polymerization.


