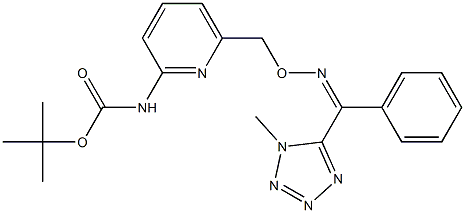
How is Picarbutrazox synthesised and what does it do?
Synthesis of Picarbutrazox
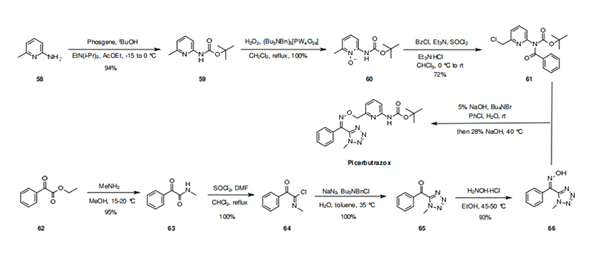
The synthesis of picarbutrazox involves the late stage combination of two advanced building blocks, the pyridine carbamate 61 and the tetrazolyloxime 66. The key compounds required for this synthesis begin with reaction of 2-aminopyridine 58 with phosgene in the presence of tert-butanol to afford carbamate 59 in 94% yield. The pyridine 59 is oxidized quantitatively to its pyridine N-oxide analogue 60, followed by a nice one pot procedure involving chlorination of the methyl group of the pyridine and protection of its NH functionality, by the successive addition of benzoyl chloride and thionyl chloride to give 61 in 72% yield. The second building block synthesis starts with the amidation of ethyl phenylglyoxylate (62) affording amide 63 in 95% yield. The tetrazole ring is prepared in two steps by chlorination of 63 with thionyl chloride, affording intermediate 64, which is subsequently treated with sodium azide in the presence of a phase transfer catalyst to yield quantitatively 65. The α-ketotetrazole is then oximated using hydroxylamine, yielding 66. The two advanced intermediates 61 and 66 are combined together in basic conditions to deliver, after debenzoylation, picarbutrazox.
Uses of Picarbutrazox
Nippon Soda presented picarbutrazox as a new oomycete specialist fungicide in 2013. This new active ingredient belongs to an unprecedented class of tetrazolyloximes for which the mode of action is, as yet, unknown. Picarbutrazox is active against P. viticola and P. infestans, and its spectrum also includes important Pythium species responsible for damping-off disease in many crops. It is theorized that picarbutrazox works by effecting the biosynthesis of phospholipids, which disrupts the normal function of the pathogen's cellular membrane.