Pyriofenone: Synthesis and Application
Synthesis of Pyriofenone
Pyriofenone is synthesised using methyl 2-chloro- 4-methyl nicotinate as a raw material by chemical reaction. The specific synthesis steps are as follows:
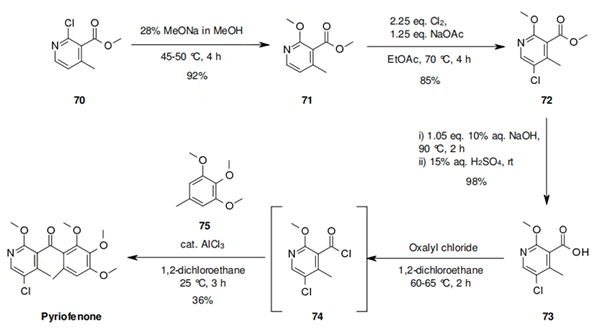
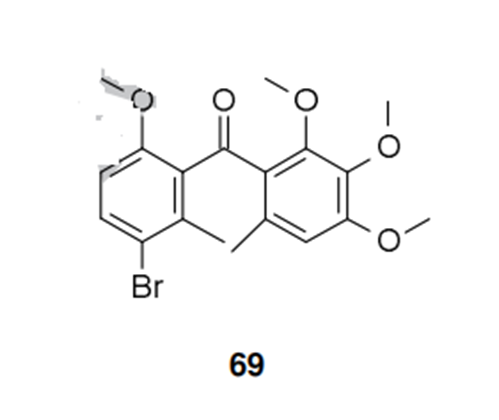
The synthesis starts with methyl 2-chloro- 4-methyl nicotinate (70) which has not been reported by ISK, but for which several syntheses can be found in the literature. An SNAr reaction allows the introduction of the methoxy group, which followed by a selective chlorination of the pyridine ring, provides compound 72 in 85% yield. Hydrolysis to the corresponding acid 73, and conversion to the acid chloride gives 74, which is primed for Friedel-Crafts acylation with 3,4,5-trimethoxy-toluene (75) under Lewis acid catalysis. The formation of the acid chloride and the Friedel-Crafts acylation have been reported as a one-pot procedure. A similar Friedel-Crafts acylation has also been used by BASF scientists for the synthesis of metrafenone (69). Other synthetic approaches to pyriofenone are reported in the original active ingredient patent.
Application of Pyriofenone
Pyriofenone is a new fungicide introduced by ISK to control powdery mildews in cereals, grapes and vegetables. The mode of action is not known but it could be from the same group of fungicides as metrafenone 69, since pathogens with cross-resistance to both compounds have been reported in the literature. In addition, their chemical structures are very closely related so it is not unreasonable to assume that they share the same mode of action. It has been proposed that the molecules are exerting their antifungal effect by disruption of actin.