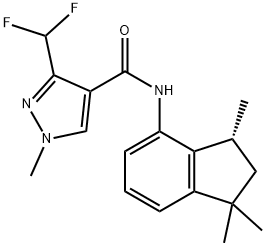
A novel anilide fungicide: Inpyrfluxam
Description
Inpyrfluxam (INDIFLIN) is a novel anilide fungicide that inhibits energy production in pathogenic fungi and is classified as a succinate dehydrogenase inhibitor (SDHI) fungicide. This amine moiety is closely related to the trimethyldihydrobenzofuraneamine of furametpyr (1). Recently, it has been shown that inpyrfluxam is cross-resistant to several other SDHIs. Inpyrfluxam has one asymmetric carbon at the 3′-position of the Indane ring, which provides R and S enantiomers. The R isomer is active as a fungicide and represents approximately 97% of the total isomer population of Inpyrfluxam. Since Inpyrfluxam exhibits high efficacy against a broad range of diseases of various crop plants, such as rice, soybeans, cereals, apples, sugar beet, and so forth, the knowledge about the environmental fate and behavior of Inpyrfluxam has been based on the so far unpublished proprietary data in submitted and released registration documents of plant protection products[1].
Synthesis method
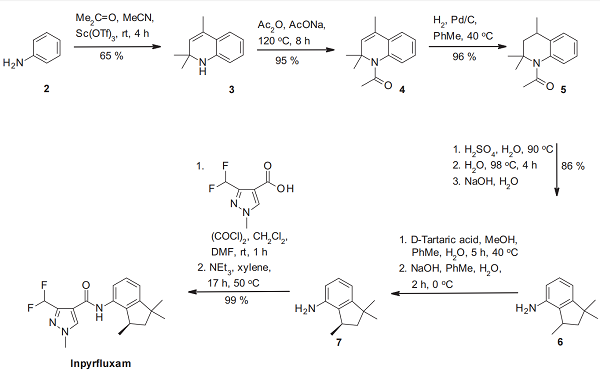
The synthesis of inpyrfluxam (I) starts with a modified Skraup cyclization of aniline (2) and acetone under lanthanide catalysis. The ring nitrogen of the resulting trimethylated 1,2-dihydroquinoline 3 is then acetylated to 4, which in turn is hydrogenated with hydrogen and palladium on charcoal to deliver the tetrahydroquinoline derivative 5. This intermediate is then converted via rearrangement under acidic conditions to 1,1,3-trimethylindan-4-amine (6). After chiral resolution of this racemic indane derivative into its (R)-enantiomer 7, this stereoisomer is then transformed by amidation with the acid chloride of the privileged SDHI acid 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid into inpyrfluxam[2].
References
[1] Takeshi Adachi, Takuo Fujisawa, Yusuke Suzuki. “Photodegradation of an Anilide Fungicide Inpyrfluxam in Water and Nitrate Aqueous Solution.” Journal of Agricultural and Food Chemistry 69 44 (2021): 12966–12973.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.