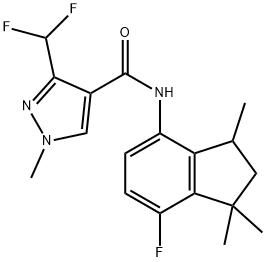
How to synthesize fluindapyr?
Overview
Fluindapyr is the latest succinate dehydrogenase inhibitor (SDHI) chiral fungicide with a broad bactericidal spectrum and good efficacy[1]. It was announced by Isagro in 2017 and is jointly developed by Isagro S.p.A. and FMC Corp. under the codes IR 9792 and F9990. This SDHI is closely related by structure to inpyrfluxam (I), the only minor differences being that fluindapyr possesses an additional fluorine atom in ring position 7 of the indane bicycle and is developed as the racemate and not, as in the case of inpyrfluxam, as a specific stereoisomer.
Uses
The research on fluindapyr mainly focused on chemical synthesis, cross-sensitivity, and the plant disease control effect by mixing fluindapyr with other pesticides. For instance, fungicide products composed of fluindapyr and phenamacril have a good control effect on rice seedling disease and various crop blight. Fluindapyr has been registered for use in maize, leafy vegetables, nuts, and forage fodder[2]. The efficacy of fluindapyr against cucumber powdery mildew and tomato gray mold was proved good in China. Therefore, fluindapyr has excellent application prospects in cucumber and tomato. At present, fluindapyr has been registered in Paraguay, America, and will be registered, sold, and applied in other countries, including China, in the future.
Synthesis method
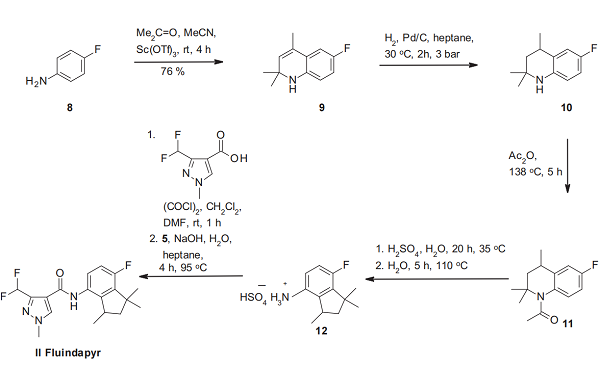
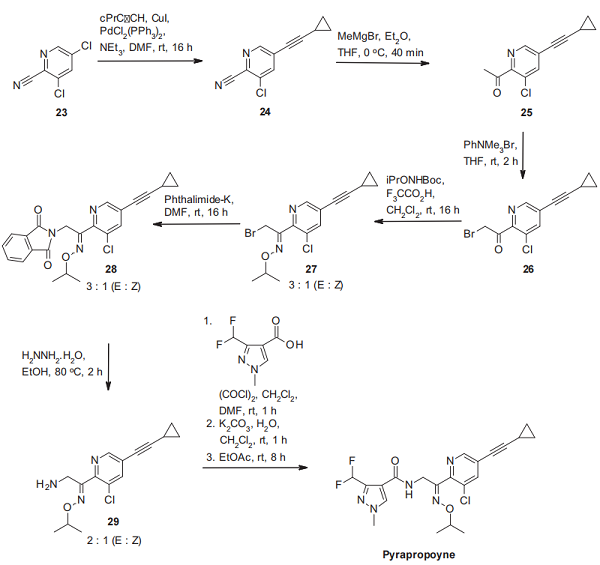
The synthesis of fluindapyr (II) can be carried out in a very similar manner for inpyrfluxam, with 4-fluoroaniline (8) being used as starting material instead of aniline (2), and the order of two steps has been interchanged[3]. In contrast to the inpyrfluxam synthesis, the Skraup cyclization product 9 is first submitted to hydrogenation of its C–C double bond, and then the resulting tetrahydroquinoline 10 is acetylated. The acetamide 11 undergoes under acidic conditions the same rearrangement as the trimethylated 7-fluoroindanylamine salt 12, which we have seen for inpyrfluxam. Finally, amidation with the well-known 3-difluoromethyl-1-methyl-1Hpyrazole-4-carboxylic acid leads to fluindapyr.
References
[1] Zhou Tong, JinSheng Duan. “Enantioselective Degradation and Bioactivity Mechanism of a New Chiral Fungicide Fluindapyr in Paddy Ecosystems.” Journal of Agricultural and Food Chemistry 71 3 (2023): 1426–1433.
[2] Peilin Guo . “Separation and determination of fluindapyr enantiomers in cucumber and tomato and by supercritical fluid chromatography tandem mass spectrometry.” Food Chemistry 395 (2022): Article 133571.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.