How to synthesis Pyrapropoyne?
Description
Nissan Chemical Industries announced Pyrapropoyne as a new fungicide in 2017. This new active ingredient also belongs to the emerging group of SDHIs, which possess an amine moiety with a C2- linker between the carboxamide function and the phenyl or pyridyl ring. The oxime function of pyrapropoyne, linked to this ethylene linker in the amine portion, has a defined Zstereochemistry.
Synthesis method
![1H-Pyrazole-4-carboxamide,N-[(2Z)-2-[3-chloro-5-(2-cyclopropylethynyl)-2-pyridinyl]-2-[(1-methylethoxy)imino]ethyl]-3-(difluoromethyl)-1-methyl- Article illustration](/NewsImg/2024-02-05/6384273873118529646694738.png)
The synthesis of pyrapropoyne starts with the palladium-catalyzed Sonogashira coupling of 2-cyano-3,5-dichloropyridine (23) with cyclopropyl acetylene, which occurs regioselectively in the 5-position of the pyridine ring[1]. The cyano group of the resulting cyclopropyl ethynyl pyridine derivative 24 is then transformed with the Grignard reagent methylmagnesium bromide into the methyl ketone 25. Subsequent α -bromination and oximation of the ketone function deliver the α-bromooxime 27 in an E / Z ratio of 3:1. The replacement of the bromide by an amino group is carried with the phthalimide intermediate 28 to yield the α -aminooxime 29 in an E / Z ratio of 2:1. Finally, amidation with 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid and isomerization of the E-isomer into the Z-form delivers pyrapropoyne.
Reference
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
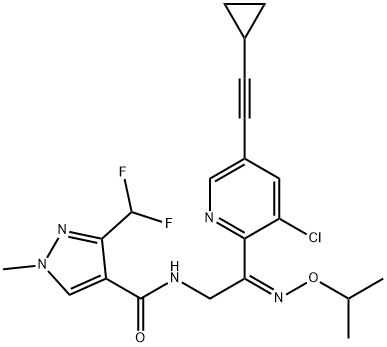 1H-Pyrazole-4-carboxamide,N-[(2Z)-2-[3-chloro-5-(2-cyclopropylethynyl)-2-pyridinyl]-2-[(1-methylethoxy)imino]ethyl]-3-(difluoromethyl)-1-methyl-
1H-Pyrazole-4-carboxamide,N-[(2Z)-2-[3-chloro-5-(2-cyclopropylethynyl)-2-pyridinyl]-2-[(1-methylethoxy)imino]ethyl]-3-(difluoromethyl)-1-methyl- 1803108-03-3