The synthesis method of Florylpicoxamid
Overview
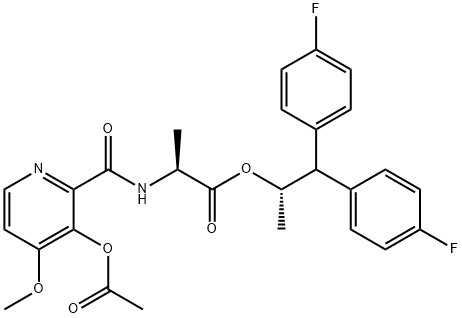
Florylpicoxamid, (S)-1,1-bis(4-fluorophenyl)propan-2-yl(3-acetoxy-4-methoxypicolinoyl)-l-alaninate, is part of a new generation of QiI fungicides developed by Corteva Agriscience, and can be seen as fully synthetic analog of fenpicoxamid and its active principle UK-2A (37). It is a second-generation picolinamide fungicide inspired by a natural product and this compound the Qi site of the mitochondrial cytochrome bc1 complex. It is currently being registered in China for the control of gray mold in a variety of crops[1].
Mechanism of action
The inherent antifungal activity of florylpicoxamid was tested on 13 fungal species, namely Zymoseptoria tritici, Cercospora sojina, Sclerotinia sclerotiorum, Magnaporthe grisea, Parastagonospora nodorum, Cercospora beticola, Colletotrichum orbiculare, Botrytis cinerea, Sclerotinia rolfsii, Ustilago maydis, Alternaria solani, Macrophomina phaseolina and Rhizoctonia solani, using microtiter plate-based growth inhibition assays. Binding assays and affinity chromatography have shown that the molecular target of florylpicoxamid is a potent antifungal inhibitor that binds to the Qi site (ubiquinone reduction site) of the Cyt b protein on MET III.
Synthesis method
![L-Alanine, N-[[3-(acetyloxy)-4-methoxy-2-pyridinyl]carbonyl]-, (1S)-2,2-bis(4-fluorophenyl)-1-methylethyl ester Article illustration](/NewsImg/2024-02-05/6384274064157505808372484.png)
A highly efficient synthesis pathway toward florylpicoxamid has been developed, which applies green chemistry principles and relies on renewable raw materials. Strecker reaction from furfural (38), available from lignocellulosic biomass, delivers the aminonitrile 39, which is further converted via aza-Achmatowicz rearrangement and subsequent bromine – methoxy exchange into the tetrasubstituted pyridine 40. Subsequent nitrile hydrolysis and reductive debromination lead to 3-hydroxy-4-methoxypicolinic acid (41). This picolinic acid is then treated with the alanine ester 44 to yield the picolinic amide 45. The alanine derivative 44 can be obtained starting from (S, S)-lactide (42), the dimer of lactic acid, a raw material available by bacterial fermentation of carbohydrates. The addition of (S, S)-lactide (42) to para-fluorophenylmagnesium bromide produces two equivalents of a diol, which is directly converted by mono-deoxygenation to the chiral alcohol 43 without any loss of optical purity. Esterification with N-Boc-protected L-alanine and subsequent removal of the Boc-group affords the amine component 44. The picolinic amide 45, obtained by amidation from 44, is already the active principle to which florylpicoxamid is converted metabolically and is responsible for its fungicidal efficacy. Its synthetic conversion to florylpicoxamid is possible via base-mediated acetylation.
References
[1] Xiong Li. “Baseline sensitivity and control efficacy of a new QiI fungicide, florylpicoxamid, against Botrytis cinerea.” Pest Management Science 78 12 (2022): 5184–5190.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.