Does the Molecule of Water Have Ionic Charge?
Water (H2O) as a whole is a neutral molecule as well as a polar molecule because it have a hydrogen bonding, which due to partial positive charge on hydrogen and negative charge on oxygen.
The ionic charge pertains to the electrical charge an ion possesses resulting from the acquisition or release of electrons. Atoms are composed of protons with a positive charge, electrons with a negative charge, and neutrons with no charge, rendering them neutral.
When an atom gains or loses electrons, it transforms into an ion, adopting a positive or negative charge. If electrons are lost, the atom becomes positively charged, as the surplus of protons outweighs electrons, leading to an overall positive charge. Conversely, if electrons are gained, the atom becomes negatively charged, as the excess of electrons over protons results in an overall negative charge.
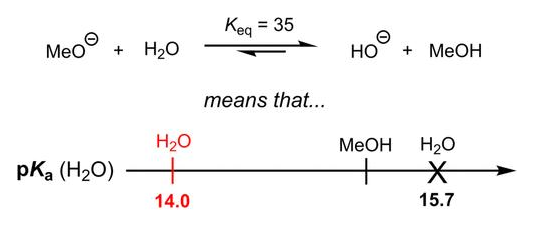
The water molecule is an electrical dipole. Charge distribution inside water molecule could be visualized as below:
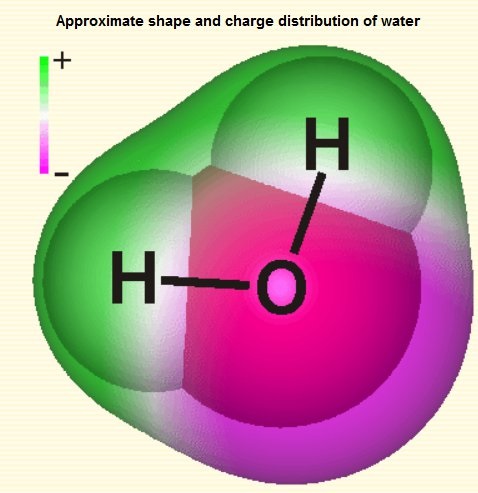
The water molecule maintains neutrality, yet it exists in equilibrium with a hydrogen cation and a hydroxide anion, each carrying a respective charge of +1 and -1. This equilibrium is governed by a constant of 10e-14, signifying that for every 10 million water molecules, there is one hydrogen cation and one hydroxide anion present.
You may like
Related articles And Qustion
See also
Lastest Price from Water manufacturers

US $1.00/ml2025-07-04
- CAS:
- 7732-18-5
- Min. Order:
- 100ml
- Purity:
- 99
- Supply Ability:
- 9999

US $120.00/kg2025-04-21
- CAS:
- 7732-18-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000