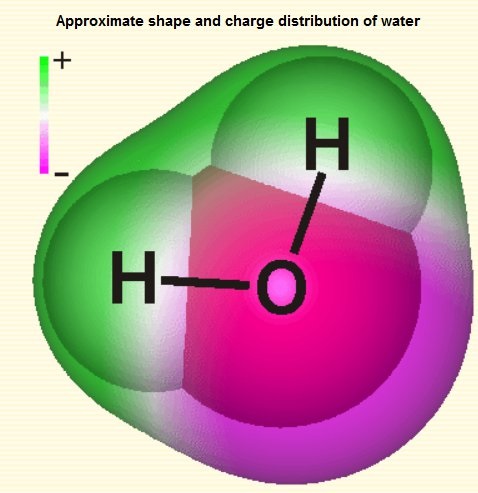
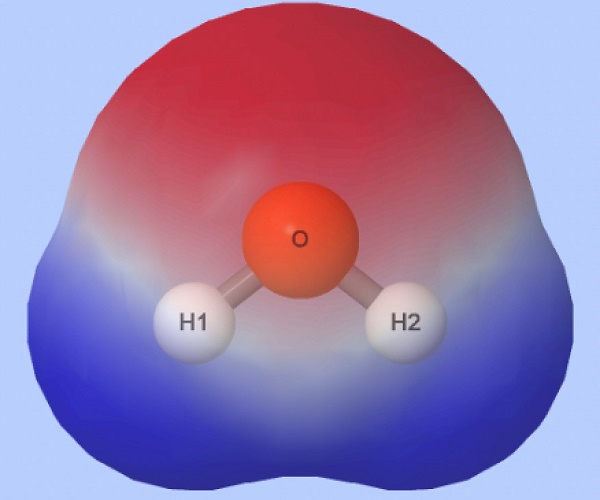
A polar molecule-water
Overview
Water plays a role in climate and weather. It is the most abundant greenhouse gas in the atmosphere, accounting for 40–70% of Earth’s heat retention. The planet’s geochemistry is linked to water cycling in seas, lakes, and rivers. All forms of life depend on water. Liquid water constitutes about half the volume of every living biological cell. Water can act as a solvent, reactant, product, catalyst, chaperone, messenger, and controller. Interactions with water are a major driving force for biomolecular structure and function in living systems. To better understand healthy life and control disease, we need to understand the relevant properties of water fully[1].
Physical and chemical properties
Water is an oxygen hydride consisting of an oxygen atom covalently bonded to two hydrogen atoms. Water is a tasteless, odorless liquid at ambient temperature and pressure. Liquid water has weak absorption bands at wavelengths of around 750 nm, which causes it to appear to have a blue color. This can easily be observed in a water-filled bath or wash basin whose lining is white. Large ice crystals, as in glaciers, also appear blue. It is an oxygen hydride, a mononuclear parent hydride, and an inorganic hydroxy compound. It is a conjugate base of an oxonium and a conjugate acid of a hydroxide.
The definition of a polar molecule
By definition, a polar molecule has a partially positive and negative end. The molecule achieves this by having an uneven distribution of electrons between its atoms. That is, electrons gather more closely to one atom than the other, thus making one atom slightly more negative. Moreover, these atoms must also be arranged so that their partial charges do not cancel each other out.
Is water polar?
Yes, water is polar because it has polar covalent bonds and is asymmetrical.
Specifically, the electronegativity of oxygen is 3.5, and the electronegativity of hydrogen is 2.1, making the difference in electronegativity between them 1.4. Atoms in polar covalent bonds generally have a difference in electronegativity between 0.4 and 1.7. Thus, the O–H bonds in water molecules—where the O end is partially negative and the H end is partially positive—are polar covalent bonds. In addition, the oxygen atom in a water molecule bonds to 2 hydrogen atoms and 2 lone pairs, giving it the steric number of 4. According to VSEPR theory, a steric number of 4 means the electron geometry of water is tetrahedral. Moreover, because that steric number includes 2 lone pairs, the molecular geometry of water is bent. A bent structure gives the water molecule its asymmetry.
References
[1] Emiliano Brini. “How Water’s Properties Are Encoded in Its Molecular Structure and Energies.” Chemical Reviews 117 19 (2017): 12385–12414.
You may like
Related articles And Qustion
See also
Lastest Price from Water manufacturers

US $1.00/ml2025-07-04
- CAS:
- 7732-18-5
- Min. Order:
- 100ml
- Purity:
- 99
- Supply Ability:
- 9999

US $120.00/kg2025-04-21
- CAS:
- 7732-18-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000