The introduction of Metyltetraprole
Description
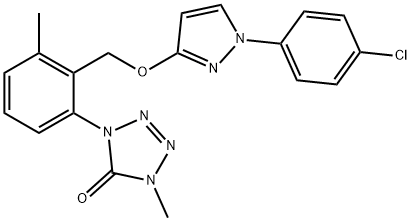
Metyltetraprole was announced by Sumitomo Chemical Company as a new fungicide in 2017 and is, just like fenpicoxamid and florylpicoxamid, an inhibitor of cytochrome bc1 (complex III of the fungal respiratory chain). It is the first member of a new generation of quinone outside inhibitor (QoI) that overcomes QoI-resistant fungi harboring G143A or F129L mutations in cytochrome b (Cytb), which are very problematic for disease management in many crops[1-2].
Metyltetraprole has precisely the same mode of action as the commercially very successful strobilurin fungicides and is closely related by structure to pyraclostrobin (46). Metyltetraprole is designed to overcome significant QoI resistance caused by a G143A mutation in the binding site of Zymoseptoria tritici, the causal agent of wheat leaf blotch, and Pyrenophora teres, the causal agent of barley net blotch. The design feature is a novel, compact tetrazolinone pharmacophore preventing a steric clash with the alanine methyl group in the mutated target site, shown below.
Synthesis method
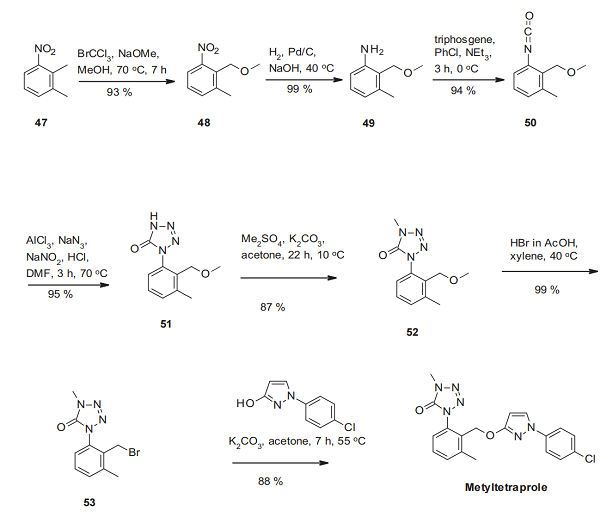
The synthesis of metyltetraprole starts with the regioselective installation of a methoxymethyl function into 2,3-dimethylnitrobenzene (47) by benzylic bromination and nucleophilic substitution[2]. The resulting nitrobenzene derivative 48 is then reduced to the corresponding aniline 49, which reacts to the isocyanate 50. This functional group's C–N double bond then undergoes a 1,3-dipolar cycloaddition with sodium azide to yield the tetrazolinone derivative 51. Methylation of one of the tetrazolinone ring nitrogen atoms affords 52, converted into the benzylic bromide derivative 53. Treatment with 1-(4-chlorophenyl)-1H-pyrazol-3-ol (already utilized as an essential building block of pyraclostrobin) affords metyltetraprole.
References
[1] Yuichi Matsuzaki. “Discovery of metyltetraprole: Identification of tetrazolinone pharmacophore to overcome QoI resistance.” Bioorganic & Medicinal Chemistry 28 1 (2020): Article 115211.
[2] Yuichi Matsuzaki. “Microtiter plate test using liquid medium is an alternative method for monitoring metyltetraprole sensitivity in Cercospora beticola.” Pest Management Science 77 3 (2020): 1226–1234.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.