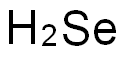The charge and toxicity of Selenium
Selenium is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic.It seldom occurs in its elemental state or as pure ore compounds in Earth's crust.

Properties
Selenium forms several allotropes that interconvert with temperature changes, depending somewhat on the rate of temperature change. When prepared in chemical reactions, selenium is usually an amorphous, brick-red powder. When rapidly melted, it forms the black, vitreous form, usually sold commercially as beads.The structure of black selenium is irregular and complex and consists of polymeric rings with up to 1000 atoms per ring. Black selenium is a brittle, lustrous solid that is slightly soluble in CS2. Upon heating, it softens at 50 °C and converts to gray selenium at 180 °C; the transformation temperature is reduced by presence of halogens and amines.
Charge on the ion formed by selenium
Electron configuration of selenium:[Ar]3d104s24p4
It's easier for selenium to accept 2 valence electrons than donate its 6 valence electrons which requires higher energy. Therefore, the ion of selenium has the charge of 2-. It's known as selenide ion.
Se can form an anion of -2 or a cation of +6. The anion is favored.
Selenium toxicity
A little bit of selenium is usually plenty to meet your daily requirements. Over the long term, routinely getting unsafe levels could lead to selenium toxicity, a condition linked to breathing issues, kidney failure, and heart problems. At high enough levels, selenium toxicity could even be fatal.
You may like
Related articles And Qustion
See also
Lastest Price from Selenium manufacturers

US $0.00/KG2025-03-05
- CAS:
- 7782-49-2
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 200 MT

US $0.00-0.00/KG2025-01-03
- CAS:
- 7782-49-2
- Min. Order:
- 10mg
- Purity:
- 99%
- Supply Ability:
- 2000tons