Charge and uses of calcium carbonate
Calcium carbonate is neutral, basically insoluble in water, and soluble in hydrochloric acid. It is one of the common substances on earth.
Uses
Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.
Charge of calcium carbonate
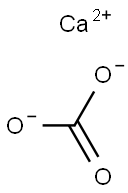
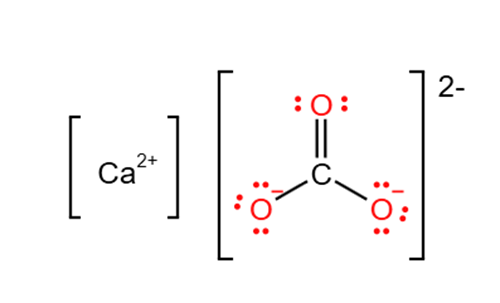
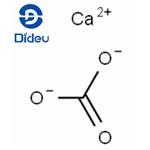
The chemical formula for calcium carbonate is CaCO3. This means that each molecule of calcium carbonate contains one calcium atom, one carbon atom, and three oxygen atoms.
To understand how the formula for calcium carbonate is derived, it's helpful to break down the composition of the compound. Calcium carbonate is made up of calcium ions (Ca2+) and carbonate ions (CO32-). The calcium ion has a positive charge, while the carbonate ion has a negative charge.
When these ions combine, they form calcium carbonate. The calcium ion has a charge of +2, while the carbonate ion has a charge of -2. To balance the charges, one calcium ion combines with one carbonate ion, resulting in a neutral compound. This is why the formula for calcium carbonate is CaCO3.
You may like
Related articles And Qustion
See also
Lastest Price from Calcium carbonate manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 471-34-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 471-34-1
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M