HBTU:Property,Reaction,Preparation and Hazard
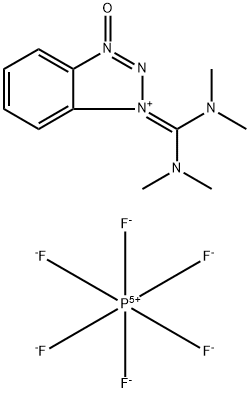
HBTU is a white crystalline solid, it is a coupling reagent commonly utilized in solid phase peptide synthesis.

HBTU Property
Crystal and solution structure studies have shown that HBTU has an aminium structure rather than a uronium structure. ( Abdelmoty, I.; Albericio, F.; Carpino, L. A.; Foxman, B. M.; Kates, S. A. Lett. Pept. Sci. 1994, 1, 57-67) HBTU generally provides high coupling yields with low racemization.For minimal racemization, HOBt can be added.
HBTU Reaction
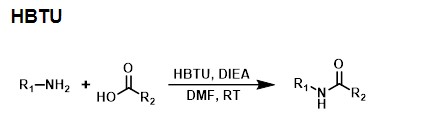
HBTU activates carboxylic acids by forming a stabilized HOBt (Hydroxybenzotriazole) leaving group. The activated intermediate species attacked by the amine during aminolysis is the HOBt ester.
To create the HOBt ester, the carboxyl group of the acid attacks the imide carbonyl carbon of HBTU. Subsequently, the displaced anionic benzotriazole N-oxide attacks of the acid carbonyl, giving the tetramethyl urea byproduct and the activated ester. Aminolysis displaces the benzotriazole N-oxide to form the desired amide.1
HBTU Uses
HBTU and HATU highly active and may be used for more difficult amide bond formations, as with resins or hindered carboxylic acids. Byproducts from HBTU and BOP-Cl are water-soluble. An amine and a carboxylic acid can be mixed with either carbodiimides or T3P for coupling without prior activation of the carboxylic acid.
HBTU Preparation
HBTU is prepared by reaction of HOBt with TCFH under basic conditions and was assigned to a uronium type structure, presumably by analogy with the corresponding phosphonium salts, which bear a positive carbon atom instead of the phosphonium residue. Later, it was shown by X-ray analysis that salts crystallize as aminium rather than the corresponding uronium salts.
HBTU Hazard
HBTU is a moderate skin sensitizer, thermal hazard analysis by differential scanning calorimetry (DSC) shows HBTU is potentially explosive.
1.Bradley, Mark; Valeur, Eric (2009-01-26). "Amide bond formation: beyond the myth of coupling reagents". Chemical Society Reviews. 38 (2): 606–631.
References:
[1] VALEUR E, BRADLEY M. Amide bond formation: beyond the myth of coupling reagents[J]. Chemical Society Reviews, 2008, 2: Page 301 to 656. DOI:10.1039/B701677H.You may like
Related articles And Qustion
Lastest Price from HBTU manufacturers

US $750.00/kg2025-08-04
- CAS:
- 94790-37-1
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 50tons

US $75.00-20.00/kg2025-04-15
- CAS:
- 94790-37-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton