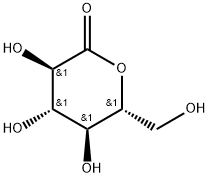
Gluconolactone: food additive
General description
D-(+)-Glucono-1.5-lactone is a naturally occurring polyhydroxy acid (PHA) with metal chelating, moisturizing and antioxidant activity. Gluconolactone can be produced by enzymatic oxidation of D-glucose oxidation. Its ability in free radicals scavenging accounts for its antioxidant property. It is used in cosmetic product and as a coagulant in tofu processing. (NCI05) Gluconolactone is a naturally-occurring food additive used as a sequestrant, an acidifier, or a curing, pickling, or leavening agent. It is a cyclic ester of D-gluconic acid. Pure gluconolactone is a white odorless crystalline powder. Gluconolactone is a Calculi Dissolution Agent. The mechanism of action of gluconolactone is as an Irrigation.
Application and Pharmacology
Dry skin, caused by improper care or genetic conditions, can affect people of all ages. Skin hydration is determined its lipid content, which inhibits water loss from the epidermis, as well as other substances such as polyhydroxy acids and gluconolactone that can bind water. The aim of this study was to evaluate skin hydration after the application of 10% and 30% gluconolactone solution in a split face model.Sixteen healthy women were qualified for the study. Three split face treatments were performed, with 10% and 30% gluconolactone solution applied to two sides of the face. Skin moisture was measured before each treatment and a week after the last treatment at three measurement sites on either side of the face, that is, on the forehead, around the eye and on the cheek. Corneometric measurements showed a significant increase in facial skin hydration after gluconolactone treatment. No significant differences were observed between the application of 10% and 30% solution.Gluconolactone is a moisturizing substance which works well in dry skin care. Gluconolactone appears to effectively moisturize the skin without causing irritation. Increasing the concentration from 10% to 30% does not increase the moisturizing effect, similarly to lactobionic acid[1].
D-(+)-Glucono-1.5-lactone was evaluated as an excipient for tablets prepared by direct compression using various drugs known to be difficult to compress. The physical properties of the tablets were evaluated after compression and after storage and were satisfactory. Comparative studies were conducted between g1uconolact.one and anhydrous lactose, a common direct compression diluent, for development of static charges during blending, flow, drug distribution, drug stratification, color distribution, compressibility, and preservation against mold growth. Gluconolactone possesses those properties necessary to produce high quality tablets by the direct compression process. Separate powdered mixtures of aspirin USP with gluconolactone, anhydrous lactose, spray-dried lactose, man-nitol, and sorbitol were stored at various humidities and temperatures for specified periods and tested for the integrity of aspirin. Gluconolactone contributed least to the degradation of the drug as compared to other excipients studied. A preliminary in uiuo study also was conducted on the bioavailability of aspirin from separate and similar mixtures with gluconolactone, anhydrous lactose, and starch. Cluconolactone did not show any inhibitory effect on aspirin absorption[2].
Synthesis
Four gold(I) complexes conceived as anticancer agents were synthesized by reacting [Au(PEt3)Cl] and [Au(PPh3)Cl] with ligands derived from δ-D-gluconolactone. The ligands’ structure was designed to combine desired biological properties previously reported for each group. Ligands were synthesized from δ-D-gluconolactone via ketal protection and hydrazide formation followed by cyclization with CS2 to produce the novel oxadiazolidine-2-thione 7 and 8. Increasing of the ligands’ lipophilicity via ketal protection proved useful since all four gold(I) complexes showed anticancer and antileishmanial properties. The IC50 values are at low micromolar range, varying from 2-3 μM for the most active compounds. The free D-gluconate 1,3,4 oxadiazole-derived ligands were neither toxic nor presented anticancer or antileishmanial properties. Triethylphosphine-derived compounds 9 and 10 were more selective against B16-F10 melanoma cell line. Although similar in vitro antileishmanial activity was observed for the gold(I) precursors themselves and their derived complexes, the latter were three times less toxic for human THP-1 macrophage cell line; this result is attributed to an isomeric variation of the D-gluconate ligand and the oxadiazole portion, which was one of the key concepts behind this work. These findings should encourage further research on gold(I) complexes to develop novel compounds with potential application in cancer and leishmaniasis chemotherapy[3].
Figure 1 Ligands were synthesized from Glucono-1.5-lactone via ketal protection and hydrazide formation
References
1.Nasir S. S., Wilken J. L. O. & Akhtar B., "Application of gluconolactone in direct tablet compression," Journal of pharmaceutical sciences, Vol.66, No.3(1977), p.370.
2.Jarząbek Perz S., Mucha P. & Rotsztejn H., "Corneometric evaluation of skin moisture after application of 10% and 30% gluconolactone," Skin Research and Technology, Vol.27, No.5(2021), pp.925-930.
3. Anticancer and antileishmanial in vitro activity of gold(I) complexes with 1,3,4-oxadiazole-
2(3H)-thione ligands derived from δ-D-gluconolactone
References
1.Nasir S. S., Wilken J. L. O. & Akhtar B., "Application of gluconolactone in direct tablet compression," Journal of pharmaceutical sciences, Vol.66, No.3(1977), p.370.
2.Jarz?bek Perz S., Mucha P. & Rotsztejn H., "Corneometric evaluation of skin moisture after application of 10% and 30% gluconolactone," Skin Research and Technology, Vol.27, No.5(2021), pp.925-930.
3. Anticancer and antileishmanial in vitro activity of gold(I) complexes with 1,3,4-oxadiazole-
2(3H)-thione ligands derived from δ-D-gluconolactone
You may like
Related articles And Qustion
Lastest Price from D-(+)-Glucono-1,5-lactone manufacturers

US $1200.00-1100.00/ton2025-08-06
- CAS:
- 90-80-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $9.00/KG2025-07-10
- CAS:
- 90-80-2
- Min. Order:
- 1000KG
- Purity:
- ≥99.0%
- Supply Ability:
- 200 tons