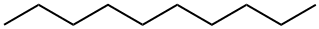Toxicological information of decane
Background
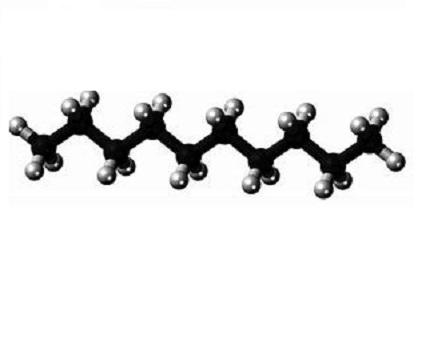
Decane refers to an alkane containing 10 carbon atoms and 22 hydrogen atoms in its molecular structure, the chemical formula is C10H22, and there are 75 isomers. Unless otherwise specified, decane generally refers to n-decane.
Preparation
1. Preparation method: In a dry reaction flask equipped with a stirrer, a thermometer, and a reflux condenser (with a calcium chloride drying tube), add 25 mL of hexamethylphosphoramide (HMPA), 2.7 g of 1-iododecane ( 0.01mol), sodium cyanoborohydride 0.943g (0.015mol). The reaction was carried out at 70 °C for 2 h with stirring. After cooling, 25 mL of water was added and extracted three times with ether. The ether extracts were combined, washed twice with water, dried over anhydrous magnesium sulfate, and fractionated under reduced pressure. The fractions at 68-79°C/1.86kPa were collected to obtain 1.25-1.3 g of n-decane with a yield of 88%-90%.
2. Preparation method: n-decane can also be prepared by reacting 23g (1.0mol) sodium metal with 75.5g (62mL, 0.5mol) 1-bromopentane or 99g (65.5mL, 0.5mol) 1-iodopentane, and collecting 171 ~174 ℃ fraction, about 28g n-decane, yield 79%. [2]
Toxicological information
1. Acute toxicity: mice inhaled LC50: 72300mg/m3/2h; mice intravenously injected LDL0: 912mg/kg;
2. Tumor: mouse skin TDL0: 25gm/kg/52W-I; [2]
3. Subacute and chronic toxicity: rats inhaled 540ppm for 18 hours a day, 7 days a week, and lasted for 57 days, which had a significant impact on body weight, and the total number of white blood cells decreased significantly, but there was no bone marrow or other obvious pathological changes. Continuous inhalation of 500 mg/m3 for 30 days and nights in a large amount, the activities of catalase, cholinesterase, and dihydroxyribonuclease in the blood were decreased, and the content of sulfhydryl groups was also decreased, and it became more significant with the exposure time.
4. Carcinogenicity: The lowest percutaneous toxic dose in mice (TDL0): 25g/kg (52 weeks, intermittent), which is positive for tumor.
Ecological data
1. Biodegradability: In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 33.2h (theoretical).
2. Bioconcentration: BCF: 143.8 (theory) [2]
3. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to the pollution of surface water, soil, atmosphere and drinking water.
Properties and stability
1. Stability: stable
2. Incompatible substances: strong oxidants, strong acids, strong bases, halogens
3. Polymerization Hazard: Non-polymerization [2]
Storage method
Storage Precautions: Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly closed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to sparks. Storage areas should be equipped with emergency release equipment and suitable containment materials. [2]
Hazard overview
Health Hazards: Harmful to the body by inhalation, oral administration or skin absorption, and its vapor or mist is irritating to eyes, skin, mucous membranes and respiratory tract. Inhalation can cause chemical pneumonitis and pulmonary edema.
Environmental Hazard: Harmful to the environment and can cause pollution to water, soil and atmosphere.
Explosion Hazard: This product is flammable and irritating.
First-aid
Skin Contact: Remove contaminated clothing and rinse skin thoroughly with soap and water.
Eye Contact: Lift the eyelids and flush with running water or normal saline. seek medical attention.
Inhalation: Quickly leave the scene to fresh air. Keep the airway open. If breathing is difficult, give oxygen. If breathing stops, give artificial respiration immediately. seek medical attention.
Ingestion; drink enough warm water to induce vomiting. seek medical attention.
Fire-fighting measures
Hazardous characteristics: flammable, its vapor and air can form an explosive mixture, which can cause combustion and explosion in case of open fire and high heat energy. It can react with oxidants. In the event of a fire, there is a risk of explosion of heated containers.
Hazardous combustion products: carbon monoxide, carbon dioxide. Move the container from the fire area to an open area as much as possible.
Extinguishing media: foam, carbon dioxide, dry powder, sand.
Extinguishing method: Water extinguishing is ineffective, but water must be used to keep the fire container cool. Protect firefighters with water mist and plug escaping liquids with sand.
Leak emergency treatment
Quickly evacuate personnel from the leaked contaminated area to a safe area, isolate them, and strictly restrict access. Cut off the source of ignition. It is recommended that emergency personnel wear self-contained positive pressure breathing apparatus and anti-static overalls. Cut off sources of leaks as much as possible. Prevent flow into restricted spaces such as sewers and flood drains. Small spills: Absorb or absorb with sand or other non-combustible materials. It can also be scrubbed with an emulsion made of a non-flammable dispersant, which is diluted and put into the waste water system. Large spills: Construct dikes or dig pits for containment. Cover with foam to reduce vapor hazards. Transfer it to a tanker or a special collector with an explosion-proof pump for recycling or disposal.
Handling and storage
Operation precautions: closed operation, full ventilation. Operators must undergo special training and strictly abide by operating procedures. It is recommended that operators wear self-priming filter respirators (half masks), safety glasses, anti-static work clothes, and rubber oil-resistant gloves. Keep away from fire and heat sources, and smoking is strictly prohibited in the workplace. Use explosion-proof ventilation systems and equipment. Prevent vapors from leaking into the workplace air. Avoid contact with oxidants. When filling, the flow rate should be controlled, and there should be a grounding device to prevent the accumulation of static electricity. When handling, it should be lightly loaded and unloaded to prevent damage to packaging and containers. [2]
Storage Precautions: Equipped with corresponding varieties and quantities of fire fighting equipment and leakage emergency treatment equipment. Empty containers should be stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly closed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to sparks. Storage areas should be equipped with emergency release equipment and suitable containment materials.
Reference
1 Van Buuren A R, Marrink S J, Berendsen H J C. A molecular dynamics study of the decane/water interface[J]. The Journal of Physical Chemistry, 1993, 97(36): 9206-9212.
Related articles And Qustion
See also
Lastest Price from Decane manufacturers

US $5.00-2.00/KG2024-10-11
- CAS:
- 124-18-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg
US $0.00/ml2023-02-24
- CAS:
- 124-18-5
- Min. Order:
- 0.1ml
- Purity:
- GC≥98%
- Supply Ability:
- 100 ml