D-(+)-Glucono-1,5-lactone: generalist in food and medicine
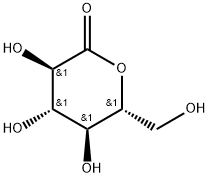
D-(+)-Glucono-1,5-lactone is abbreviated as lactone or GDL, and its molecular formula is C6Hl0O6. It has been proved by the toxicological test of Shandong Provincial Institute of Labor Hygiene that it is a non-toxic edible substance. It is white crystal or white crystalline powder, almost odorless, sweet first and then sour, and easily soluble in water. D-(+)-Glucono-1,5-lactone is used as a coagulant, mainly used in tofu production, and can also be used as a protein coagulant for dairy products.

Uses
Coagulant: D-(+)-Glucono-1,5-lactone is mainly used in tofu production. The tofu made has good water retention, fine texture, smooth and delicious taste, and has a preservation effect. It can also be used as a protein coagulant for dairy products, used in the production of yogurt, cheese, etc.
Preservative: It has a preservative effect on mold and general bacteria, and can be used to preserve fish, meat, poultry, shrimp, etc., so that the products do not change color while maintaining the elasticity of the meat.
Bulking agent: It can be mixed with sodium bicarbonate to make baking powder. Gluconic acid reacts with sodium bicarbonate to generate carbon dioxide when heated. It has good swelling effect and no odor. It is especially suitable for cakes, fried foods, etc.
Acidifier: It can be used for fruit juice drinks, jellies, etc. It can also be mixed with sodium bicarbonate to make high-end beverages. It not only has strong gas production, but also can slowly hydrolyze gluconic acid, which is refreshing and delicious without irritating the stomach.
Chelating agent: D-(+)-Glucono-1,5-lactone can also be used in grape juice or other berry juice wines. Adding this product can prevent the formation of tartar. It can be used in dairy products to prevent the formation of milkstone.
Precautions
D-(+)-Glucono-1,5-lactone is a white powdery crystal. It can be stored for a long time under dry conditions, but it is easy to decompose into acid in a humid environment, especially in an aqueous solution. At room temperature, the lactone in the solution is partially decomposed into acid within 30 minutes. The hydrolysis rate is accelerated above 65 degrees, and it is quickly and completely converted into gluconic acid above 95 degrees. Therefore, when using D-(+)-glucono-1,5-lactone as a coagulant, it must be dissolved in cold water and used up within half an hour, and the aqueous solution must not be stored for a long time.
You may like
See also
Lastest Price from D-(+)-Glucono-1,5-lactone manufacturers

US $1200.00-1100.00/ton2025-08-06
- CAS:
- 90-80-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $9.00/KG2025-07-10
- CAS:
- 90-80-2
- Min. Order:
- 1000KG
- Purity:
- ≥99.0%
- Supply Ability:
- 200 tons