Gefitinib vs Erlotinib vs Afatinib: differences in anticancer drugs
Gefitinib vs Erlotinib vs Afatinib
What is Gefitinib?
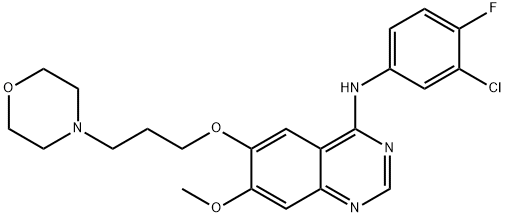
Gefitinib (ZD1839) is a medicine for the treatment of non-small cell lung cancer (NSCLC) that has spread to surrounding tissues (locally advanced) or to other parts of the body. It is an orally active selective inhibitor of epidermal growth factor receptor tyrosine kinase, an enzyme that regulates intracellular signalling pathways involved in the proliferation and survival of cancer cells. In NSCLC cell lines and xenografts, gefitinib dose-dependently inhibits cell proliferation and tumour growth and potentiates the cytotoxic effects of chemotherapy and/or radiotherapy.

In patients with advanced NSCLC who had failed one or two prior chemotherapies, gefitinib 250 or 500mg once daily induced an objective response in approximately 19% of patients in a double-blind trial (n = 210). In another double-blind trial including 216 patients with NSCLC who had failed two or more prior chemotherapies, gefitinib 250 or 500mg once daily induced an objective response in 11.8 and 8.8% of patients, respectively; approximately 40% showed an improvement in disease-related symptoms. Gefitinib was generally well tolerated and the most common adverse events were mild skin rashes and diarrhoea.
What is Erlotinib?
Erlotinib is a drug used to treat certain small cell lung cancers or advanced metastatic pancreatic cancers. The FDA originally approved erlotinib for the treatment of NSCLC in November 2004. According to the American Society of Clinical Oncology (ASCO), erlotinib is recommended as a first-, second-, or third-line treatment for advanced NSCLC, depending on patient characteristics. Erlotinib is recommended as a first-line agent only if the patient has a known EGFR mutation (first-line therapy for NSCLC). Erlotinib may be used as second-line therapy if there is no treatment response after four cycles of therapy or if disease progression occurs after or following platinum-based first-line therapy. The guidelines state that erlotinib may be used as third-line therapy if disease progression occurs and the patient has not been treated with erlotinib and gefitinib. The third-generation tyrosine kinase inhibitor ositinib prolonged overall survival in patients with EGFR-mutated advanced non-small cell lung cancer compared with erlotinib.
Erlotinib is a reversible first-generation receptor tyrosine kinase inhibitor (along with gefitinib) acting primarily on the epidermal growth factor receptor (EGFR), a member of the ErbB receptor family. The drug interacts with both wild-type and mutation EGFR. The ErbB family can form homodimers or heterodimers, which are often implicated in downstream effects and pathogenesis of many types of carcinomas studied in humans. Receptor tyrosine kinase inhibitors (TKI) prevent the phosphorylation of their substrate in the cell signaling pathway. EGFR normally plays a role in many cellular functions, including differentiation, proliferation, and angiogenesis, all of which are hallmarks of cancer.
Erlotinib is available as 25 mg, 100 mg, and 150 mg oral tablets.The recommended starting dose for NSCLC is 150 mg per day, while the standard starting dose for pancreatic cancer is 100 mg per day.Patients are advised to take erlotinib on an empty stomach as studies have shown that bioavailability is increased when taken with food. Patients should avoid concomitant use of proton pump inhibitors (PPIs) while taking erlotinib as the higher gastric pH may alter erlotinib concentrations. The adverse effects of erlotinib are similar to those of other drugs in the EGFR TKI family, most notably diarrhoea and rash. Chest pain is also common.
What is Afatinib?
Afatinib is a treatment for locally advanced or metastatic non-small cell lung cancer (NSCLC) with non-resistant EGFR mutations or resistance to platinum-based chemotherapy. Afatinib is a targeted therapy that irreversibly inhibits the ErbB family of tyrosine kinases. It is an FDA-approved first-line therapy for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) carrying non-drug-resistant epidermal growth factor receptor (EGFR) mutations. It has been reported as a second-line treatment for advanced squamous non-small cell lung cancer (NSCLC).
Afatinib is also a tyrosine kinase inhibitor with a mechanism of action that involves irreversible inhibition of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and HER4.EGFR and HER2 are part of the family of receptor tyrosine kinases that play an important role in tumour cell proliferation and tumour angiogenesis. These receptors are known to exhibit overexpression in many types of cancer cell types. Afatinib also has activity against T790 mutations that are insensitive to its standard therapy. Afatinib exerts its anti-tumour effect in head and neck squamous cell carcinoma (HNSCC) by inhibiting mTORC1, which initiates apoptosis in cancer cells.
Afatinib is an oral medication available only in tablet form. It should be taken on an empty stomach at least 1 hour before or 2 hours after a meal, as absorption is reduced by high-fat meals. The recommended dose is 40 mg tablets daily in patients with normal creatinine and without any significant hepatic dysfunction until disease progression or until no longer tolerated by the patient. Following oral administration, the time to peak plasma concentration (Tmax) has been reported to be between 2 and 5 hours. Afatinib has a predictable and manageable side effect profile, with diarrhoea and rash/acne being the most common adverse events, accounting for 88% and 82% of cases, respectively.
Differences in anticancer drugs
Gefitinib, erlotinib and afatinib are all anticancer drugs widely used in the treatment of advanced non-small cell lung cancer (NSCLC) with proven efficacy. They all belong to the tyrosine kinase inhibitor (EGFR TKI) class of drugs. However, all three have slightly different indications, dosages and adverse effects. Based on reported data, afatinib is not superior to erlotinib in the treatment of EGFR-mutant NSCLC. However, afatinib has been found to be more effective than erlotinib in the treatment of advanced squamous cell carcinoma (as second-line therapy).
References:
[1] Culy CR, Faulds D. Gefitinib. Drugs. 2002;62(15):2237-48; discussion 2249-50. doi: 10.2165/00003495-200262150-00008. PMID: 12381224.
[2] Carter J, Tadi P. Erlotinib. [Updated 2022 Dec 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554484/
[3] Moosavi L, Polineni R. Afatinib. [Updated 2022 Jul 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542248/
You may like
Related articles And Qustion
Lastest Price from Gefitinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 184475-35-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/g2025-04-21
- CAS:
- 184475-35-2
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g