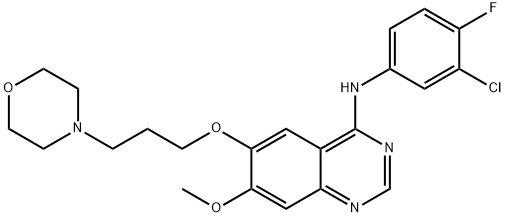
Gefitinib: Pharmacodynamic Properties, Dosage and Administration
General Description
Gefitinib is a medication that has been studied for its pharmacodynamic properties and antitumor effects in non-small cell lung cancer (NSCLC). It inhibits downstream signal transduction pathways involved in cell growth, proliferation, survival, and angiogenesis. It disrupts the expression of c-fos mRNA, inhibits mitogen-activated protein kinase (MAPK) activity, and induces G1 cell cycle arrest. It also inhibits the Akt/NF-κB pathway and reduces the production of angiogenic factors. In NSCLC, gefitinib inhibits the growth of cancer cells with high epidermal growth factor receptor (EGFR) expression and shows synergistic effects with radiation and certain chemotherapeutic agents. It enhances tumor regression and sensitizes tumors to radiation. The recommended dosage is 250mg once daily, which has been proven effective and well-tolerated. Dosage adjustment may be required in patients with hepatic impairment, and caution should be exercised with drugs affecting the CYP3A4 enzyme system. Overall, gefitinib shows promise as a treatment option for NSCLC patients, particularly those with high EGFR expression.
Figure 1. Tablets of gefitinib
Pharmacodynamic Properties
Effects on Downstream Signal Transduction
Gefitinib is a medication that has been studied for its effects on downstream signal transduction. In ex vivo A431 tumor xenograft samples, gefitinib was found to inhibit the expression of c-fos mRNA, a gene involved in cell growth and proliferation. The inhibition of c-fos mRNA was observed 6 hours after dosing and lasted for approximately 36 hours. Gefitinib also demonstrated inhibitory effects on mitogen-activated protein kinase (MAPK) activity in human cancer cell lines that overexpress epidermal growth factor receptor (EGFR). This inhibition was associated with a decrease in the number of proliferating cells and a reduction in MAPK expression. Furthermore, gefitinib disrupted the regulation of cyclin-dependent kinase 2, which is involved in cell cycle progression. It induced a G1 cell cycle arrest and upregulated the cyclin-dependent kinase inhibitor p27Kip1. Gefitinib was also found to inhibit the antiapoptotic Akt/nuclear factor-kappa B (NF-κB) pathway. It reduced the phosphorylation of Akt and I-κB, and inhibited NF-κB transcription activity. In addition, gefitinib showed antiangiogenic effects by inhibiting the production of angiogenic factors such as vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) in cancer cell lines. It also reduced tumor-induced vascularization in animal models. Overall, gefitinib has been shown to interfere with various signaling pathways involved in cell growth, proliferation, survival, and angiogenesis. These findings contribute to our understanding of the molecular mechanisms underlying the therapeutic effects of gefitinib in cancer treatment. 1
Antitumor Effects of Gefitinib in Non-Small Cell Lung Cancer
Gefitinib is a medication that has been studied for its antitumor effects in non-small cell lung cancer (NSCLC). In vitro studies have shown that gefitinib can inhibit the growth of NSCLC cell lines with varying levels of epidermal growth factor receptor (EGFR) expression. The inhibitory effect was dose-related, with IC50 values similar to those observed in high EGFR-expressing cell lines. Additionally, gefitinib has demonstrated synergistic or additive interactions with radiation and certain chemotherapeutic agents in these cell lines. Combination treatments with gefitinib and vinorelbine or paclitaxel showed synergistic effects, while combinations with cisplatin had additive to antagonistic effects. Furthermore, gefitinib has shown antiproliferative effects in NSCLC cell lines resistant to cisplatin and docetaxel. In vivo studies using mouse models of NSCLC have further supported the antitumor effects of gefitinib. Treatment with gefitinib led to dose-dependent tumor growth inhibition, especially at the maximum tolerated dose. Additionally, gefitinib potentiated the inhibitory effects of chemotherapy agents, with some combinations resulting in tumor regression. Gefitinib was also shown to enhance the sensitivity of NSCLC tumors to radiation. In summary, gefitinib has exhibited antitumor effects in NSCLC, both in vitro and in vivo. It has shown growth inhibition, increased apoptosis, and enhanced the efficacy of radiation and certain chemotherapy agents. These findings suggest that gefitinib may be a promising treatment option for NSCLC patients, particularly those with high EGFR expression. Further research is needed to explore its full potential and determine optimal treatment regimens. 2
Dosage and Administration
Gefitinib is administered orally at a dosage of 250mg once daily for patients with inoperable or recurrent non-small cell lung cancer (NSCLC), as approved in Japan. This dosage has been proven to be equally effective as the 500 mg/day dosage, while causing fewer grade 3/4 adverse events, based on findings from two phase II trials. Additionally, phase I clinical data indicates that dosages of up to 700 mg/day are generally well tolerated in patients with NSCLC or other tumor types. It has been suggested that dosage adjustment is not necessary in patients with mild to moderate hepatic impairment, according to the results of one pharmacokinetic analysis. Furthermore, it's important to note that drugs affecting the CYP3A4 enzyme system may impact the metabolism of gefitinib. Therefore, caution should be exercised when administering gefitinib concurrently with drugs that affect the CYP3A4 enzyme system. Overall, the recommended dosage and administration of gefitinib are based on its efficacy and tolerability in patients with NSCLC, with considerations for hepatic impairment and potential drug interactions. 3
Reference
1. Albanell J, Codony-Servat J, Rojo F, et al Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001; 61(17):6500-6510
2. Sirotnak FM, Zakowski MF, Miller VA, et al. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000; 6(12):4885-4892.
3. Culy CR, Faulds D. Gefitinib. Drugs. 2002;62(15):2237-2250.
Related articles And Qustion
Lastest Price from Gefitinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 184475-35-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/g2025-04-21
- CAS:
- 184475-35-2
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g