Puerarin in the root of kudzu: pharmacokinetics and adverse effects
General Description
Puerarin is a flavonoid extracted from the root of Pueraria lobata, which has been shown to have anti-diabetes, anti-tumor, and phytoestrogenic activity. However, its low bioavailability has limited its clinical utilization. Various preparation technologies have been developed to improve its clinical efficacy. Puerarin's pharmacokinetics in rats after oral administration is characterized by rapid absorption, quick elimination, and minimal accumulation. Puerarin has shown no significant toxicity or adverse effects when administered orally, but caution should be exercised during pregnancy. Adverse reactions have been reported following puerarin injection. Understanding the pharmacokinetics of puerarin is crucial for optimizing its therapeutic use and developing effective drug delivery systems.

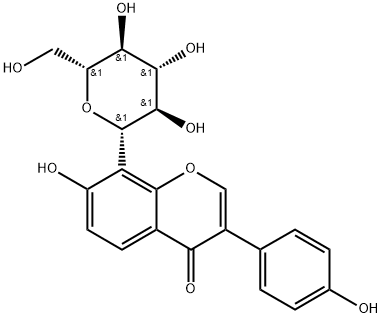
Figure 1. Puerarin
Puerarin in the root of kudzu
Puerarin is a flavonoid extracted from the dried root of Pueraria lobata, also known as Kudzu root. This plant is rich in isoflavones and has been shown to have various pharmacological properties, including anti-diabetes, anti-tumor, and phytoestrogenic activity. Puerarin is the most important flavonoid isolated from Pueraria lobata and is the main active ingredient responsible for its cardiovascular effects. The Chinese Pharmacopoeia requires a minimum of 2.4% puerarin content in Pueraria lobata. However, the low bioavailability of puerarin due to its poor lipid and water solubility, incomplete absorption, and fast elimination has limited its clinical utilization. To address this issue, researchers have developed various preparation technologies, including structural modification studies, derivatives, nanotechnologies, microemulsions, nanocrystals, and dendrimers, to improve the clinical efficacy of puerarin. Despite its limitations, accumulated data suggests that puerarin has beneficial effects on cardiovascular diseases, including systemic and arterial hypertension, and coronary artery disease. In conclusion, the extraction and development of puerarin from Pueraria lobata holds great potential for the treatment of cardiovascular diseases and other medical conditions. 1
Pharmacokinetics
The pharmacokinetics of puerarin has been studied extensively in rats after oral administration. It is characterized by rapid absorption, quick elimination, and minimal accumulation in the body. Various absorption models, both in vitro and in vivo, have shown that puerarin has low permeability in the intestines, with less than 2% cumulative absorption within four hours. The primary mechanism of absorption is passive diffusion, aided by P-glycoprotein-mediated active transport, and there may be an intestinal first-pass effect. Puerarin is rapidly absorbed from the intestines into the bloodstream after oral administration, with approximately 24.6% plasma protein binding rate. It can be detected in various organs, particularly those with rich blood flow, such as the kidney, liver, lung, heart, and spleen. Puerarin can cross the blood-brain barrier and the placental barrier but in relatively low amounts. Its distribution in the body can be influenced by different forms of administration. In terms of metabolism, puerarin is quickly metabolized to daidzein, possibly through microbial metabolism in the large intestine. In the liver, puerarin undergoes hydrolysis, reduction, sulfation, and glucuronidation reactions before entering circulation. The metabolites of puerarin, along with the unchanged drug, are primarily excreted through urine, feces, and bile. The excretion route may vary depending on the dosage and route of administration. Overall, understanding the pharmacokinetics of puerarin is crucial for optimizing its therapeutic use and developing effective drug delivery systems. 2
Adverse effects
Puerarin has been extensively studied for its potential therapeutic effects. However, it is essential to consider the adverse effects associated with its use. When administered orally, puerarin has shown no significant toxicity or adverse effects. In a study conducted on Sprague-Dawley rats, oral administration of 250 mg/kg puerarin daily for 28 days did not cause any significant alterations in histological, biochemical, and hematological parameters. However, caution should be exercised when using puerarin during pregnancy. Studies have demonstrated that puerarin can cross the placental barrier, leading to high concentrations in the plasma of fetal rats. This has been associated with decreased embryonic development and viability. Therefore, pregnant women are advised to use puerarin with caution. In some cases, adverse reactions have been reported following puerarin injection. These include acute intravascular hemolysis and allergic reactions. It is important to note that these reactions may be attributed to individual patient differences and variations in production processes among different manufacturers of herbal injections in China, due to the imperfect quality standards. In summary, while puerarin has shown no significant toxicity or adverse effects when administered orally, caution is warranted during pregnancy. 2
Reference
1. Fusi F, Trezza A, Tramaglino M, Sgaragli G, Saponara S, Spiga O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol Res. 2020;152:104625.
2. Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28(7):961-975.
Related articles And Qustion
See also
Lastest Price from Puerarin manufacturers

US $1200.00-1100.00/ton2025-10-17
- CAS:
- 3681-99-0
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00/kg2025-04-28
- CAS:
- 3681-99-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg