Bemotrizinol: A New Sunscreen Active Ingredient considered by FDA
General Description
Bemotrizinol is an organic UV filter used in sunscreens to absorb UV-A rays, offering protection against skin aging and certain types of skin cancer. Its enhanced fat solubility allows for easy incorporation into cosmetic oils, improving its efficacy and broad-spectrum activity against UV-B rays as well. Additionally, bemotrizinol is known for its photostability, remaining effective under sunlight exposure. Commercially available under names like Tinosorb S and Escalol S, bemotrizinol is being considered by the U.S. FDA for inclusion in over-the-counter sunscreen products. The FDA's OTC sunscreen monograph currently lists a limited number of approved UV filters, hindering innovation in sun protection products available to American consumers. DSM, a company, is seeking FDA approval for bemotrizinol's inclusion, based on its strong safety profile and effectiveness. If approved, bemotrizinol would expand the options for formulating safe and effective sun protection products, addressing the current limitations in the variety of UV filters available for use in sunscreen formulations in the United States.

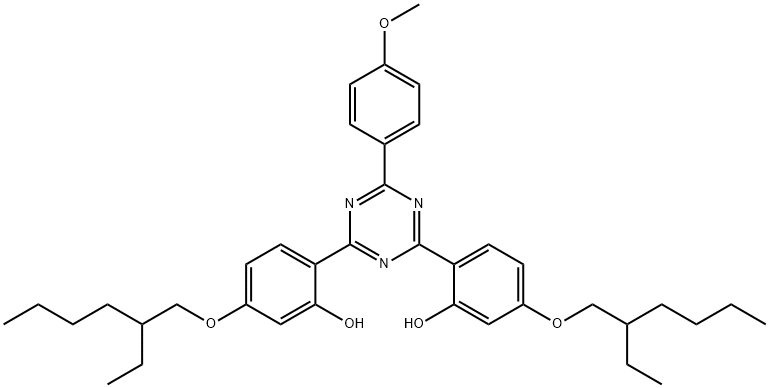
Figure 1. Bemotrizinol
Properties in UV Filter
Bemotrizinol, also known as bis-ethylhexyloxyphenol methoxyphenyl triazine, is an organic UV filter commonly used in over-the-counter sunscreen products. It is a member of the methoxybenzenes family. Bemotrizinol primarily absorbs UV-A rays, which are responsible for skin aging and some types of skin cancer. One key property of bemotrizinol is its increased fat solubility compared to older broad-spectrum chemical agents. This means that it can dissolve easily in cosmetic oils, enhancing its efficacy and broad-spectrum activity. The oil solubility allows better incorporation into sunscreen formulations and enhances its ability to protect against both UV-A and UV-B rays. Another notable property of bemotrizinol is its claimed photostability. This means that it remains stable under exposure to sunlight, prolonging its effectiveness in providing protection against UV-rays. Photostability increases the onset of action and overall efficiency of bemotrizinol when applied topically. Bemotrizinol is commercially available under different names, such as Tinosorb S and Escalol S. These products are widely used in sunscreens to provide effective protection against harmful UV radiation and reduce the risk of skin damage and sunburn. 1
Active Ingredient considered by FDA
Bemotrizinol is a new sunscreen active ingredient that is currently being considered by the U.S. Food and Drug Administration (FDA). Nonprescription sunscreen drugs are regulated by the FDA and are used topically to help prevent sunburn, decrease the risk of skin cancer, and prevent early skin aging caused by exposure to ultraviolet (UV) radiation. Compared to other countries, such as those in Europe, the U.S. has fewer approved UV filters for use in sunscreen products. Currently, the FDA's OTC sunscreen monograph lists only 16 UV filters, with around 10 considered suitable for formulating effective and broad-spectrum sunscreens. This limited number of approved UV filters restricts the variety of innovative sun protection products available to American consumers. To address this issue, DSM, a company, is seeking FDA approval for the inclusion of bemotrizinol in the OTC sunscreen monograph. Bemotrizinol has been used globally since 2000 as a UVA and UVB light absorber, and it has a strong safety profile based on extensive nonclinical tests. The FDA had previously indicated that bemotrizinol could be considered for inclusion pending the submission of additional data to support its Generally Recognized as Safe and Effective (GRASE) determination. Bemotrizinol is a high molecular weight, photostable, oil-soluble UV filter that offers broad-spectrum protection. It is more soluble in cosmetic oils, which makes it easier to formulate effective sunscreens. The FDA requires comprehensive safety studies, including clinical and nonclinical assessments, to determine the GRASE status of sunscreen ingredients. These studies evaluate potential adverse effects, bioavailability, pharmacokinetics, and post-marketing safety information. Bemotrizinol is the first new sunscreen active ingredient to be evaluated under the FDA's revised GRASE and Maximal Usage Trials (MUsT) testing guidelines for OTC substances. Its approval would expand the options for formulating safe and effective sun protection products for long-term use. 2
Reference
1. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 135487856, Bemotrizinol.
2. D'Ruiz CD, Plautz JR, Schuetz R, et al. Preliminary clinical pharmacokinetic evaluation of bemotrizinol - A new sunscreen active ingredient being considered for inclusion under FDA's over-the-counter (OTC) sunscreen monograph. Regul Toxicol Pharmacol. 2023;139:105344.
Related articles And Qustion
Lastest Price from Bemotrizinol manufacturers
US $0.00-0.00/kg2025-08-20
- CAS:
- 187393-00-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/KG2025-07-05
- CAS:
- 187393-00-6
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS