Gefitinib: Uses in non-small cell lung cancer (NSCLC)
Imatinib mesylate is a novel tyrosine kinase inhibitor and a derivative of phenylpyrimidine. About 95% of patients with chronic myeloid leukemia (CML) are Philadelphia chromosome-positive, that is, the proto-oncogene ABL of chromosome 9 is ectopic to the oncogene of chromosome 22 called the breakpoint clustering region (BCR). The two genes are recombined to produce the fusion protein p-210. Compared with the normal C-ABL protein p-150, p-210 has higher tyrosine kinase activity, which can stimulate leukocyte proliferation and lead to leukemia. Imatinib mesylate has a strong inhibition on the activity of ABL tyrosine kinase both in vitro and in vivo, specifically inhibit the expression of ABL and the proliferation of BCR-ABL cells, and thus can be used in the the treatment of CML. In addition, this drug also inhibits tyrosine kinases of platelet-derived growth factor (PDGF) and stem cell factor (SCF) receptors, and inhibits PDGF and SCF-mediated biochemical reactions, but does not affect the signal transduction of other stimulating factors such as epidermal growth factor.
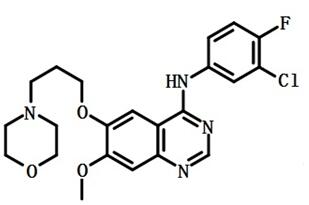
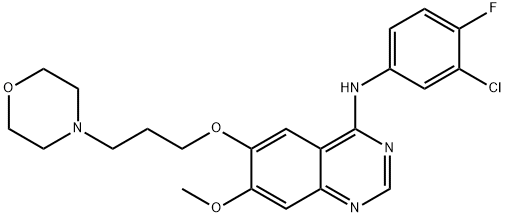
Figure 2: Structural formula of Gefitinib
Gefitinib was introduced in Japan as a daily oral monotherapy for the treatment of inoperable or recurrent non-small cell lung cancers (NSCLC). This anilinoquinazoline derivative can be synthesized in 6 steps starting from 6,7-dimethoxyquinazolin-4(3H)-one by successive monodemethylationlacetylation of the 6-hydroxy-group followed by chlorination and reaction with 3-chloro-4-fluoroaniline, finally deacetylation and alkylation with 3-(4-morpholinyl)propylbromide complete the synthesis. Gefitinib reversibly inhibits the activity of the epidermal growth factor receptor tyrosine kinase (EGRF TK). This inhibits autophosphorylation of EGRF and blocks the cascade of intracellular events which have been implicated in the proliferation, survival and metastasis of cancer cells.
Gefitinib diplays good selectivity for the EGRF TK relative to other growth factors in human umbilical endothelial cells. It is similarly selective relative to other kinases, for example cerB2. Data from two large phase II studies in patients with pretreated NSCLC have shown that gefitinib induces a response rate approaching 20% in patients receiving the agent as a second line therapy and approximately 10% in those pretreated with more lines of chemotherapy. Gefitinib has good bioavailability and is metabolized in the liver via the cytochrome P450 3A4 enzyme system with a mean elimination half life of 28 h. Gefitinib has been generally well tolerated in cancer patients with predominant side effects being acne-like skin-rash, diarrhea, nausea, vomiting and mild to moderate myelosuppression.
References
U.S. Environmental Protection Agency. Technology transfer network air toxics Web site. 1999. Acrylonitrile. Hazard summary created in April 1992. Revised in January 2000 (updated 2007).
Proctor, N. H., Hughes, J. P., and Fishman, M. L., eds. 1988. Chemical hazards of the work place, 2nd ed. Philadelphia, PA: Lippincott.
Budavari, S., ed. 1989. The Merck index. An encyclopedia of chemicals, drugs, and biologicals, 11th ed. Rahway, NJ: Merck and Co. Inc.
Material Safety Data Sheet. 2001. Acetic anhydride. Mallinnckrodt Baker, Inc., New Jersey (updated 2007).
You may like
Related articles And Qustion
See also
Lastest Price from Gefitinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 184475-35-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/g2025-04-21
- CAS:
- 184475-35-2
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g