Fmoc-Lys(Boc)(Me)-OH: Applications in Synthesis of Histone Tail Peptides and its Concise Preparation Method
General Description
Fmoc-Lys(Boc)(Me)-OH, a pivotal component in peptide synthesis, features protective structures ensuring efficient assembly. Derived from fluorenylmethoxycarbonyl, the Fmoc group safeguards the amino functionality, simplifying purification. Lysine contributes its epsilon-amino group, shielded by the Boc group, ensuring stability. Additionally, the terminal methyl modification enhances stability and specificity. This derivative allows precise integration into peptides, ensuring sequence accuracy. Particularly in synthesizing histone tail peptides, Fmoc-Lys(Boc)(Me)-OH facilitates epigenetic studies with high yields and versatility. Its synthesis involves strategic methods enabling convenient preparation and modification. Notably, peptides incorporating methylated lysines exhibit resistance to enzymatic degradation, enhancing stability in biological assays. A concise preparation method involves a one-pot reaction sequence, yielding high purity. Utilized in solid-phase peptide synthesis, it enables the synthesis of site-specifically lysine monomethylated peptides. In summary, Fmoc-Lys(Boc)(Me)-OH streamlines peptide synthesis, offering new avenues for exploring histone modifications in epigenetics with exceptional fidelity and efficiency.

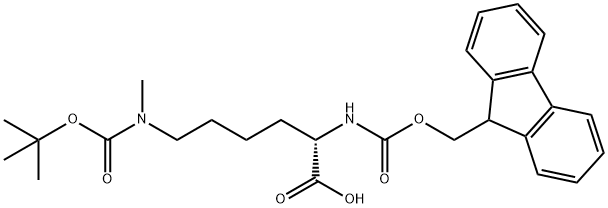
Figure 1. Fmoc-Lys(Boc)(Me)-OH
Overview
Fmoc-Lys(Boc)(Me)-OH is a crucial component in peptide synthesis, featuring a protective structure conducive to efficient assembly. The Fmoc group, derived from fluorenylmethoxycarbonyl, acts as a safeguard for the amino functionality, simplifying purification processes during peptide formation. Lysine, an essential amino acid, contributes its epsilon-amino group, shielded by the Boc (tert-butoxycarbonyl) group, ensuring chemical stability throughout synthesis. Moreover, the terminal methyl modification (Me) enhances stability and specificity, reinforcing the molecule's integrity. This modified lysine derivative allows precise integration into peptides, ensuring accuracy in sequence and structure. Consequently, Fmoc-Lys(Boc)(Me)-OH serves as a cornerstone in peptide chemistry, facilitating the creation of intricate and biologically active peptides with exceptional fidelity. Its strategic design streamlines the synthesis process, enabling researchers to construct peptides with desired functionalities for various biomedical and pharmaceutical applications. 1
Applications in Synthesizing Histone Tail Peptides
Fmoc-Lys(Boc)(Me)-OH, a derivative of Nε-methyl-L-lysine, holds significant promise in synthesizing histone tail peptides, crucial for epigenetic studies. Its synthesis involves a strategic method employing dicyclohexylcarbodiimide and 7-amino-4-methylcoumarin in dichloromethane. This yields a high product yield of 90%. Moreover, the procedure offers versatility, enabling the acylation of nitrogen nucleophiles by carboxylic acids. This methodology allows for the convenient preparation of Fmoc-Lys(Boc)(Me)-OH and related compounds like Nα-Fmoc-Nε-dimethyl-L-lysine. The process relies on malonate derivatives and dibromobutane to generate key intermediates, which can be modified to introduce desired groups at the ε-position. Notably, Fmoc-protection renders these compounds suitable for both solution and solid-phase peptide synthesis. Furthermore, peptides incorporating methylated lysines, facilitated by Fmoc-Lys(Boc)(Me)-OH, exhibit resistance to enzymatic degradation by trypsin and lysyl endopeptidase. This attribute enhances their stability and utility in biological assays and therapeutic applications. In summary, the synthesis of Fmoc-Lys(Boc)(Me)-OH streamlines the production of methylated peptides crucial for studying epigenetic modifications. Its robustness, versatility, and compatibility with various synthesis methods mark it as a significant advancement in peptide chemistry, offering new avenues for exploring histone modifications and their functional implications. 2
Concise Preparation Method
The concise preparation method of Fmoc-Lys(Boc)(Me)-OH involves a one-pot reaction sequence. Initially, Nα-Fmoc-Nε-(benzyl, methyl)-lysine hydrochloride is subjected to consecutive reductive benzylation and reductive methylation in the presence of TEA and Boc anhydride in anhydrous dichloromethane. The reaction progress is monitored, and upon completion, Pd-C catalyst is removed, and the filtrate is sequentially washed to yield a crude product. This crude product is then purified via silica gel column chromatography using a methanol and dichloromethane eluent, resulting in pure Fmoc-Lys(Boc)(Me)-OH. The obtained Nα-Fmoc-Nε-(Boc, methyl)-lysine is confirmed through various spectroscopic techniques, including ESI-MS, 1H-NMR, and 13C-NMR, showing characteristic signals and mass peaks. The final product appears as a white powder with a high yield of 93%, melting point ranging from 85-87°C, and specific rotation [α]20 D 1:7 0:2. Fmoc-Lys(Boc)(Me)-OH is then utilized in the synthesis of site-specifically lysine monomethylated peptides. By incorporating Nα-Fmoc-Nε-(Boc, methyl)-lysine as a building block via solid-phase peptide synthesis, a peptide containing monomethylated lysine is successfully synthesized. This method offers a streamlined approach for the preparation of Fmoc-Lys(Boc)(Me)-OH, facilitating its application in peptide synthesis. 3
Reference
1. Boc-Lys(Fmoc)-OMe. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 56776966.
2. Chi H, Islam MS, Nsiama TK, Kato T, Nishino N. A convenient preparation of N (ε)-methyl-L-lysine derivatives and its application to the synthesis of histone tail peptides. Amino Acids. 2014; 46(5): 1305-1311.
3. Huang ZP, Du JT, Su XY, Chen YX, Zhao YF, Li YM. Concise preparation of N(alpha)-Fmoc-N(epsilon)-(Boc, methyl)-lysine and its application in the synthesis of site-specifically lysine monomethylated peptide. Amino Acids. 2007; 33(1): 85-89.