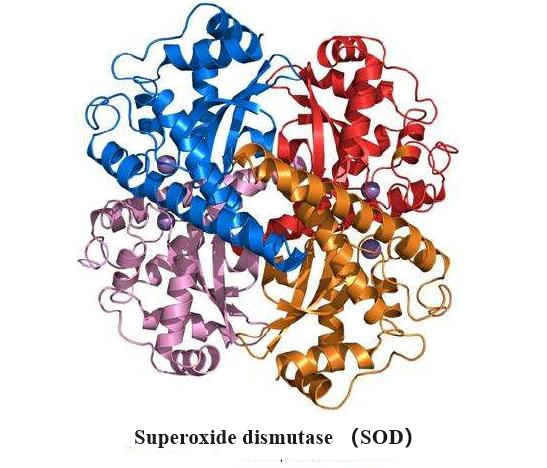
What is the structure of superoxide dismutases?
Introduction
Superoxide dismutase (SOD) is a group of antioxidant enzymes that catalyze the dismutation of superoxide to oxygen and hydrogen peroxide. SODs are critical antioxidative proteins, protecting the host from oxidative damage. Three classes of SOD have evolved with distinct protein folds and different catalytic metal ions: Cu, ZnSODs, MnSOD/FeSODs, and NiSODs.
The first superoxide dismutase (SOD) to be demonstrated in mammalian tissues was CuZn-SOD. The protein was previously known as haemocuprein or erythrocuprein. It has Mr about 33000 and comprises two equal non-covalently bound subunits. It possesses 2Cu and 2Zn atoms/molecule. Later, the Mn-SOD type, first discovered in prokaryotes, was found in chicken and mammalian tissues. Mammalian Mn-SODs have Mr just above 80000 and are composed of four equal noncovalently bound subunits. They contain 2-4Mn atoms/molecule. The CuZn-SODs and the MnSODs do not show similarities in protein structure[1].
Structure of Cu, ZnSODs
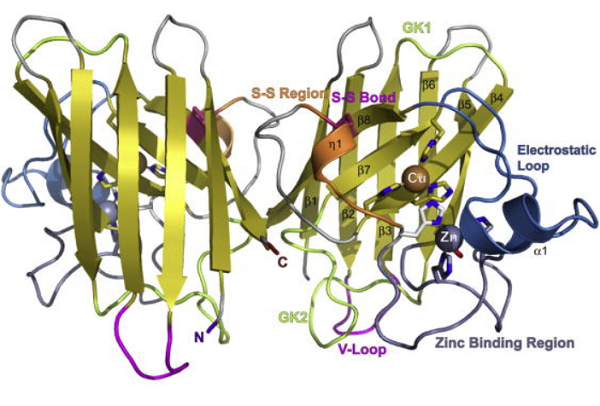
Cu, ZnSODs are composed of two identical subunits related by a two-fold symmetry axis. It is a dimeric metallo-protein containing one Zn2+ and one Cu2+ ion per subunit, which dismutates the superoxide anion O2− into O2 and H2O. Each subunit consists of a β-barrel composed of eight antiparallel β-strands arranged in a Greek key motif. Tightly packed hydrophobic residues form the core of the barrel, and loops β3/β4 and β6/β7 form the +3 β-strand Greek critical connections (GK1 and GK2). Two conserved Leu residues within these Greek key loops fill the ends of the β-barrel and are termed the cork residues. Two other primary external loops form the active site channel. The first, the β4/β5 loop, tethers the dimer interface with the active site zinc and contains a stabilizing intervening disulfide bond that aids stability. The disulfide stabilizes both the subunit fold and the dimer interface. The second, β7/β8 or electrostatic loop (EL), guides and accelerates the substrate O2·− into the active site. The evolution of Cu, ZnSOD dismutase, and the Greek key β-barrel, as well as the functional roles of the various residue positions, was defined by structural analyses, allowing functional equivalences to be identified. Furthermore, the identification of electrostatic guidance in Cu, ZnSOD opened the door to examining electrostatic guidance in other transient interactions involving electron transfer, as illustrated by computational analyses of plastocyanin with cytochrome c. Overall, the stable Greek critical scaffold supports elements for electrostatic guidance, dimer formation, and active site metallochemistry[2].
The Cu and Zn sites are positioned outside the β-barrel in the active site channel; the metal ions tie structural elements together and provide catalytic roles. Furthermore, hydrophobic anchors tie the β-barrel to the active site Cu ion. The active site in each Cu, ZnSOD subunit contains one Cu ion ligated by three histidines when in the reduced state and one Zn ion ligated by one aspartic acid and three histidines, whose side chains all reside outside of the β-barrel. One of the Zn ion ligands' histidine ligands also ligates the Cu ion when in the oxidized state and thus has been termed the bridging histidine. The exposed Cu ion is additionally coordinated by a water molecule and the substrate/product/reaction intermediate/water depending upon the particular reaction step. Importantly, steric selection allows superoxide into the active site but not larger anions such as phosphate. Of interest, while the bridging histidine has been shown to have different conformations depending on the redox state of the active site, more recent advances have revealed that the copper ion can occupy two positions in a single crystal.
Structure of MnSOD/FeSODs
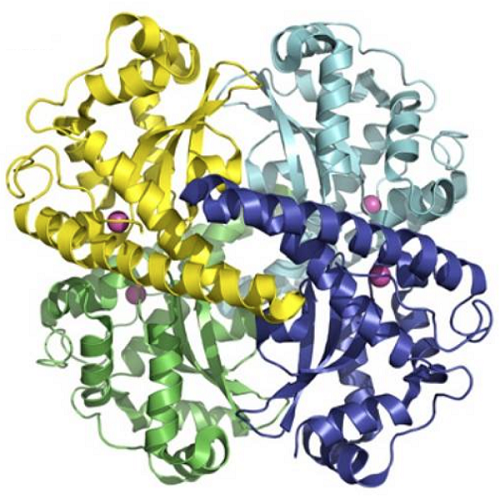
Structural studies help define the molecular mechanisms behind catalysis and product inhibition of MnSOD and FeSOD, which first achieved success in the late 1980s and early 1990s. In the Mn/FeSOD structures, the polypeptide chain is divided into N-terminal helices and a C-terminal α/β domain. The N-terminal domain mediates multimerization, with most bacterial structures forming dimers. Human MnSOD (hMnSOD) differs by assembling into a tetramer by forming a dimer of dimers that creates two symmetrical four-helix bundles. Notably, the I58T polymorphism in hMnSOD modifies Ile58, the largest contributor to buried surface area in the tetramerization interface. Structural biochemistry studies revealed that the I58T polymorphism causes packing defects, and it significantly reduces the thermal stability of hMnSOD, resulting in rapid inactivation at temperatures elevated above normal that are associated with fever or inflammation. However, epidemiology studies have focused not on the I58T but on the V16A and A9V polymorphisms of the MnSOD precusor that disrupts targeting of MnSOD to mitochondrial.
The active site of Mn/FeSOD is located between the N and C-terminal domains, and it differs from that of Cu and ZnSOD by containing a single metal ion. The metal ion is coordinated in a strained trigonal bipyramidal geometry by four amino acid side chains, His26, His74, Asp159, and His163 (human sequence), and by one solvent molecule, as observed in the human structure. The superoxide reaches the active site through a funnel that uses electrostatics for guidance and has a narrow entrance to the active site, limiting access to only small ions. Also at this funnel vertex are residues His30 and Tyr34, which function as gatekeeper residues, specifically blocking access to the metal ion, with computational analysis suggesting that Tyr34 moves to allow passage of the superoxide to the metal center.
References
[1] Marklund, S L. “Properties of extracellular superoxide dismutase from human lung.” Biochemical Journal 220 1 (1984): 269–72.
[1] J.J.P. Perry . “The structural biochemistry of the superoxide dismutases.” Biochimica et biophysica acta. Proteins and proteomics 1804 2 (2010): Pages 245-262.
You may like
Related articles And Qustion
See also
Lastest Price from Superoxide dismutase manufacturers

US $6.00/kg2025-04-21
- CAS:
- 9054-89-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/KG2025-04-21
- CAS:
- 9054-89-1
- Min. Order:
- 1KG
- Purity:
- 95% up by SDS-PAGE analysis
- Supply Ability:
- 20 tons