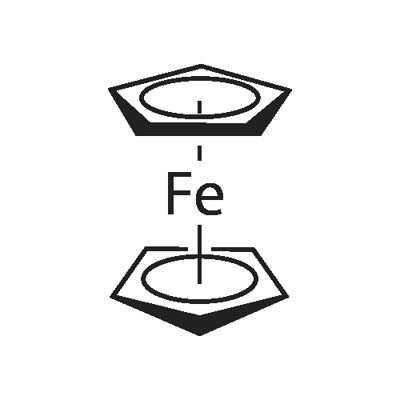
Ferrocene: An Iconic Organometallic Compound
Ferrocene is an aromatic organic transition metal compound. It is an orange-yellow powder at room temperature with a camphor smell. The melting point is 172-174 degrees, the boiling point is 249 degrees, and it can sublime above 100 degrees. It is insoluble in water, soluble in organic solvents such as benzene, ether, gasoline, and diesel, and does not react with acids, alkalis, and ultraviolet rays. It has stable chemical properties and does not decompose within 400 degrees. Its molecules are polar, have high thermal stability, chemical stability, and radiation resistance, and are widely used in industries such as industry, agriculture, medicine, aerospace, energy conservation and environmental protection.

Figure 1 Characteristics of Ferrocene
Related applications
Ferrocene itself is not very useful, but a wide variety of derivatives can be synthesized using known methods, which greatly expands the application range of ferrocene.
Anti-seismic agent
Ferrocene and its derivatives are anti-seismic agents in gasoline, which are much safer than tetraethyl lead that was once used. Gasoline additives containing ferrocene can be purchased at Halfords in the UK, and are particularly suitable for vehicles that were previously designed to use tetraethyl lead anti-seismic agents. The iron decomposed from ferrocene is deposited on the surface of the spark plug, enhancing the thermal conductivity of the spark plug. Adding ferrocene can also reduce the smoke emitted by diesel vehicles.
Medicine
Some ferrocene salts have anticancer activity, such as ferrocene analogs of tamoxifen. The mechanism is that tamoxifen can bind to estrogen, and its cytotoxicity can kill cancer cells.
Materials
Ferrocene is easy to sublimate and can be used to deposit certain fullerenes or carbon nanotubes. Aldehydes and phosphonates undergo Wittig reactions in the presence of sodium hydroxide to produce vinyl ferrocene. This compound is polymerized to produce a polymer similar to polystyrene, in which the phenyl group is replaced by a ferrocenyl group.
Ligand chirality
Ferrocene phosphine ligands are used in some transition element-catalyzed reactions, and such reactions are used industrially to synthesize drugs and pesticides. Bis(diphenylphosphino)ferrocene (dppf) is an important ligand in organic synthesis, and the mechanism of many coupling reactions is based on the formation of its palladium complex.
Preparation method
It can be obtained by heating iron powder and cyclopentadiene to 300℃ in a nitrogen atmosphere, or by reacting anhydrous ferrous chloride with sodium cyclopentadiene in tetrahydrofuran. It can also be obtained by electrolytic synthesis. Ferrocene can be synthesized using cyclopentadiene, ferrous chloride and diethylamine as raw materials. Under stirring, anhydrous ferric chloride (FeCL3) is added to tetrahydrofuran in batches, and then iron powder is added. The mixture is heated under reflux for 4.5 hours under nitrogen protection to obtain a ferrous chloride solution. The solvent tetrahydrofuran is evaporated under reduced pressure to obtain a nearly dry residue. Under ice bath cooling, a mixture of cyclopentadiene and diethylamine is added and vigorously stirred at room temperature for 6-8 hours. Excess amine is evaporated under reduced pressure, and the residue is extracted with petroleum ether under reflux. The extract is filtered while hot, and the solvent is evaporated to obtain crude ferrocene. It is obtained by recrystallization with pentane or cyclohexane, or by sublimation extraction. The yield of the refined product is 73-84%.
You may like
Related articles And Qustion
Lastest Price from Ferrocene manufacturers

US $1.00/g2025-06-02
- CAS:
- 102-54-5
- Min. Order:
- 1g
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $5.00/kg2025-04-21
- CAS:
- 102-54-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000