Application of ferrocene in chemical reactions
Introduction
Ferrocene is an organic transition metal compound with aromatic properties[1]. Its chemical formula is Fe(C5H5)2. It is an orange-yellow powder at room temperature and has a camphor odor. Melting point 172℃-174℃, boiling point 249℃, sublimation above 100℃; insoluble in water, soluble in benzene, ether, gasoline, diesel oil and other organic solvents. It does not interact with acids, alkalis and ultraviolet rays, and its chemical properties are stable and will not decompose within 400 °C. Its molecules are polar, with high thermal stability, chemical stability and radiation resistance. The application of ferrocene itself is not many, but a wide variety of derivatives can be synthesized by known methods, which greatly extends the ferrocene. Scope of application of iron. It has a wide range of applications in industry, agriculture, medicine, aerospace, energy saving, environmental protection and other industries.

Picture 1 Ferrocene powders
Structure and properties of ferrocene
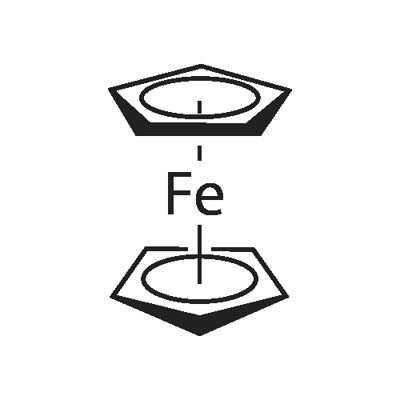
Ferrocene, also known as dicyclopentadienyl iron[2], is an organometallic compound with the chemical formula Fe(C5H5)2, orange crystalline solid; odor similar to camphor; melting point 172~174℃, sublimation above 100℃, boiling point 249℃; diamagnetic, zero dipole moment; insoluble in water, 10% sodium hydroxide and hot concentrated hydrochloric acid , soluble in dilute nitric acid, concentrated sulfuric acid, benzene, ether, petroleum ether and tetrahydrofuran. Ferrocene is stable in the air, has the effect of strongly absorbing ultraviolet rays, is quite stable to heat, and can withstand high temperature heating at 470 °C; it neither dissolves nor decomposes in boiling water, 10% boiling alkali solution and concentrated hydrochloric acid boiling solution. Ferrocene is the most important metallocene complex and the first sandwich complex to be discovered, containing two cyclopentadiene rings bonded to iron atoms.
The structure of ferrocene is that an iron atom is located between two parallel cyclopentadiene rings. In the solid state, the two locene rings are staggered to form a fully staggered configuration, and rotate relative to each other around the vertical axis when the temperature increases. The chemical properties of ferrocene are stable, similar to aromatic compounds. The ring of ferrocene can undergo electrophilic substitution reactions, such as mercurization, alkylation, acylation and other reactions. It can be oxidized to [Cp2Fe]+, and the increase of the oxidation state of the iron atom makes the electrons of the locene ring (Cp) flow to the metal, which hinders the electrophilic substitution reaction of the ring. Ferrocene is resistant to hydrogenation and does not react with maleic anhydride. Ferrocene reacts with n-butyllithium to form monolithium ferrocene and dilithium ferrocene. The ferrocene rings can interact with each other in the ferrocene molecule, and the blunting on one ring causes the other ring to be blunted to different degrees, and the degree is lighter than that on the benzene ring.
Ferrocene is prepared by heating iron powder and cyclopentadiene in a nitrogen atmosphere at 300°C, or by reacting anhydrous ferrous chloride with sodium cyclopentadiene in tetrahydrofuran. Ferrocene can be used as a rocket fuel additive, an antiknock agent for gasoline, a curing agent for rubber and silicone resin, and a UV absorber. The vinyl derivatives of ferrocene can undergo ethylenic polymerization to obtain metal-containing high polymers with carbon chain skeletons, which can be used as outer coatings for spacecraft.
Application in chemical reactions
Ferrocene is not suitable for catalytic hydrogenation, nor does it undergo Diels-Alder reaction as a diene, but it can undergo Friedel-Crafts acylation and alkylation reactions.
Ferrocene has the remarkable characteristics of an aromatic compound and can react with electrophiles to form substituted derivatives of ferrocene. Most types of substitution are 1-substituents, 1,1'-disubstituents and 1,2-disubstituents. For example, ferrocene reacts with aluminum trichloride and Me2NPCl2 in hot heptane to form dichloroferrocene phosphine, which when reacted with phenylphosphine dichloride under similar conditions gives P,P-dichlorobis(ferrocene) )-P-phenylphosphine. Similar to anisole, ferrocene reacts with P4S10 to form Dithiadiphosphetane disulfide. Ferrocene cannot be directly nitrated and halogenated because oxidation will occur first.
Ferrocene can undergo Friedel-Crafts reactions, for example, when phosphoric acid is used as a catalyst, ferrocene reacts with acetic anhydride or acetyl chloride to form acetylferrocene. Although on the one hand many reactions of ferrocene are similar to the corresponding reactions of aromatic hydrocarbons, on the other hand some reactions are clearly dominated by iron atoms.
The proton in ferrocene can be quickly extracted with butyllithium, and the product is 1,1'-dilithium ferrocene, which is a strong nucleophile. It reacts with selenium diethyldithiocarbamate, and the resulting product is sterically hindered, and two cyclopentadienyl ligands are connected by a selenium atom. This product can be generated by thermal ring-opening polymerization (ROP) to generate ferrocene polyselenide, and the corresponding polymer can also be synthesized by a similar reaction of silicon and phosphorus.
Ferrocene is easily oxidized to blue paramagnetic ferrocene ion in acidic solution, and its potential is about 0.5V based on a saturated calomel electrode. Because the product ferrocene is less reactive and easy to isolate, this ion is sometimes used as an oxidant, in the form of hexafluorophosphate or fluoroborate. Different substituent groups on the ring will change the potential value: electron-withdrawing groups (such as carboxyl groups) increase the electrode potential value; while electron-donating groups (such as methyl groups) make this value decrease, and oxidation becomes easy. The salt [Fe(η5-C5Me5)2][tcne] (tcne=tetracyanoethylene) formed by the oxidation of permethyl-substituted ferrocene has unusual magnetic properties and is a dark green crystal with alternating cations and anions long chain.
Reference
1 Yuan Yaofeng, Ye Suming, Zhang Yunwen, etc. Ferrocene derivatives with biological (physical) activity. 1995
2 Deng Zifeng, Zhu Zhongliang, Yang Zhengwei, etc. Determination of ascorbic acid by ferrocene modified green pepper seed carbon paste electrode. 1999
Related articles And Qustion
See also
Lastest Price from Ferrocene manufacturers

US $1.00/g2025-06-02
- CAS:
- 102-54-5
- Min. Order:
- 1g
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $5.00/kg2025-04-21
- CAS:
- 102-54-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000