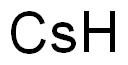Facts About Cesium
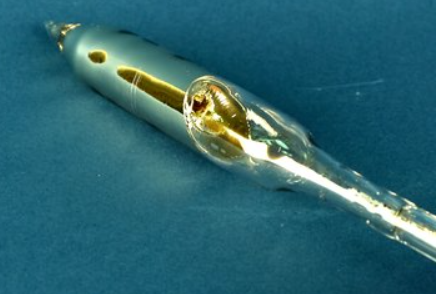
Cesium is a rare, silver-white, shiny metal with brilliant blue spectral lines; the element's name comes from "caesius," a Latin word meaning "sky blue." It is the softest metal, with a consistency of wax at room temperature. It would melt in your hands — if it didn't explode first, as it is highly reactive to moisture.

Characteristics
Cesium is a soft, malleable, silvery-white element when freshly cut and has the highest density (1873 kg.m–3) of the alkali metals, just slightly greater than that of magnesium. Like the other alkali metals, it has a body-centered cubic crystal lattice structure. Owing to its low melting point (m.p. 28.4°C), it can be liquid at room temperature.
It decomposes water vigorously, evolving hydrogen and forming the caustic cesium hydroxide, CsOH, and it even reacts with ice at temperatures above –116°C. It ignites spontaneously in oxygen and hence is difficult to handle because it reacts spontaneously in air. Therefore, it must be kept immersed in mineral oil. It is readily soluble in liquid and anhydrous ammonia, giving deep blue solutions. Moreover, cesium hydroxide is the strongest base known.
Like the other alkali metals it combines vigorously with mercury, forming a stable cesium amalgam. Cesium is the most reactive of the alkali metals with nitrogen, carbon, and hydrogen. Cesium salts color the nonluminous flame of a Bunsen gas burner reddish violet or bluish purple (460 nm). Cesium is a monoisotopic element, and hence the only naturally occurring nuclide 133Cs.
Just the facts
Atomic number (number of protons in the nucleus): 55
Atomic symbol (on the periodic table of elements): Cs
Atomic weight (average mass of the atom): 132.90
Density: 1.086 ounces per cubic inch (1.879 grams per cubic centimeter)
Phase at room temperature: solid
Melting point: 83.1 degrees Fahrenheit (28.4 degrees Celsius)
Boiling point: 1,239.8 F (671 C)
Number of natural isotopes (atoms of the same element with a different number of neutrons): 1. There are also at least 39 artificial isotopes created in a lab.
Most common isotopes: Cs-133 (100 percent of natural abundance)
History
Cesium was the first element to be discovered with a spectroscope. It was discovered in 1860 by German chemists Robert Bunsen and Gustav Kirchhoff when they were analyzing the spectrum of mineral water, according to WebElements.
The first practical applications of cesium were realized in the 1920s, according to the USGS. Cesium was used in vacuum tubes to remove traces of remaining oxygen due to its ready nature to bond with it, and as a coating on heated cathodes to increase the electric current. In later decades, more uses for cesium arose, including photoelectric cells, spectrometers and catalysts for organic reactions. The high cost of cesium and the growing popularity of similar and cheaper technologies using other alkali metals reduced the use of cesium to a handful of applications.