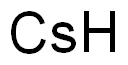The applications and Preparation of Cesium
Cesium, or Caesium, chemical symbol Cs, atomic number 55, and relative atomic mass (i.e., atomic weight) 132.90545, is the fifth alkali metal of group IA of Mendeleev’s periodic chart. The word cesium comes from the Latin word caesius, meaning blue sky, owing to the very deep blue color of its chief spectra line. Cesium is a soft, malleable, silvery-white element when freshly cut and has the highest density (1873 kg.m–3) of the alkali metals, just slightly greater than that of magnesium. Like the other alkali metals, it has a body-centered cubic crystal lattice structure.

Applications
Cesium, owing to its low electronic work function, is extensively used for manufacturing photocathode materials for photoelectric cells used in photomultiplier tubes. Cesium salts are used in moderate quantities in the manufacture of spectrometer prisms made of high-purity CsCl, CsBr, and CsI, in infrared signaling lamps, as X-ray screen phosphors, in gamma scintillation counters such as a single monocrystal made of thallium-doped CsI(Tl), in spectrophotometer lamps, and finally as getter for the final evacuation of radio and television vacuum low-voltage electron tubes to fix residual gases such as oxides, nitrides, and hydrides.
At one time the second, unit of time, was defined on the vibrational frequency 9,192,769 MHz of hyperfine transition between electronic levels of the cesium-133 atom and it is therefore used in atomic clocks. The radionuclide, cesium-137, with a half-life of 33 years, is a beta emitter, used extensively in radiotherapy as a gamma source. It is produced by fission in power nuclear reactors and, therefore, essentially recovered from spent nuclear fuel and wastes.
On the other hand, cesium has been recently evaluated for power systems for spacecraft applications as a potential rocket fuel for interplanetary travel. Actually, owing to its low work function, the cesium atom can be easily ionized thermally. The cations could then be accelerated to great speeds; hence cesium fuel can provide extraordinarily high specific impulses for rocket propulsion.
Cesium also has application in thermionic converters that generate electricity directly within nuclear reactors. Another potentially large application of cesium metal is in the production of the lowest melting-point Na-K-Cs eutectic alloy (see sodium).
Preparation
The direct metallothermic reduction of pollucite ore with sodium metal is the primary commercial source of cesium metal. In the process, raw pollucite ore is reduced with sodium molten metal at ca. 650°C to form a sodium-cesium alloy containing some rubidium as impurity. Fractional distillation of this alloy in a distillation column at ca. 700°C produces 99.9 wt.% pure cesium metal.
Cesium can also be obtained pyrometallurgically reducing the chloride CsCl with calcium metal or the hydroxide CsOH with magnesium metal. Nevertheless, the electrolytic recovery of a cesium amalgam from an aqueous solution of cesium chloride can be achieved in a process similar to the chlor-alkali production with a mercury cathode. Afterwards, the cesium is removed from the amalgam by vacuum distillation.