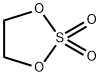
ETHYLENESULFATE: Sythesis and Application
Ethylenesulfate (ES) is utilized as an electrolyte additive in lithium ion batteries (LIBs), forming Li-ion complexes that evolve with lithium salt concentration, transitioning from fully solvated tetrahedral-like structures to contact ion-pairs and ionic aggregates.

Synthesis of ethylenesulfate
General procedure: Install a thermometer in a 2L 4-neck low-temperature cooling jacket reaction vessel, 100 g of ethylene glycol (hereinafter also referred to as a starting material) as a compound represented by Formula 1 was added at 25° C. and stirred.To the obtained solution, 1.1 equivalents of chlorine sulfide (SOCl2) as a cyclizing agent, based on 1 equivalent of ethylene glycol, were slowly added at 25° C. over 60 minutes to prepare a cyclized compound. At this time, the temperature did not exceed 40 .The solution obtained in the first reaction step was cooled to 10° C. (corresponding to the 2-1 temperature condition among the second temperature conditions), and 1100 g of methylene chloride was added thereto.When the solution was cooled to 0 to 10° C. (corresponding to the 2-2 temperature condition), an aqueous sodium bicarbonate solution (concentration of 5.3 wt %) was added to maintain the pH of the solution containing the cyclized compound of 7 to 9.The solution was cooled to 2° C. (corresponding to the 2-3 temperature condition), and then 0.0005 equivalent of ruthenium chloride was added based on 1 equivalent of ethylene glycol and stirred.While the temperature was maintained at -5 to 5°C, 1 equivalent of sodium hypochlorite was added based on 1 equivalent of the ethylene glycol, and the reaction solution temperature was maintained at less than 5°C.As a reaction termination reagent, 0.1 equivalent of hydrogen peroxide was added based on 1 equivalent of ethylene glycol, stirred for 30 minutes, and then layer separation was performed. The separated filtrate was filtered using a Celite filter, and the reaction solution was concentrated and recrystallized to obtain a solid product.[1]
Application: Ethylenesulfate as film formation additive
In the past few decades, lithium-ion batteries (LIBs) have developed as a promising power source for electric vehicles (EVs) and plug-in hybrid electric vehicles (HEVs) because of their high energy density and long life cycle. [2] Scientists found that ethylene sulfate and ethylene sulfite (ES) have similar structure, which suggests that ethylenesulfate may be a promising additive. In addition, the LUMO energy (0.11 eV) of ethylenesulfate is lower than ethylene carbonate (EC) (0.95 eV) according to our quantum chemical calculation results, which means it is easier to be reduced at the graphite anode. However, it was not reported so far to our best knowledge. To understand the compatibility between the electrolyte containing ethylenesulfate and graphite, ethylenesulfate has been studied as an electrolyte additive for AG half battery with 1 mol L−1 LiPF6/EC + DMC + EMC (1:1:1, w:w:w) electrolyte.
Ethylene sulfate is investigated as a novel film formation electrolyte additive for graphite anode material in lithium-ion battery. The CV results reveal that ethylenesulfate is reduced prior to ethylene carbonate (EC) at the interface between graphite and electrolyte, while it cannot prevent the sustained reduction of propylene carbonate (PC) when the amount of ethylenesulfate is lesser than 3 wt% in the PC-based electrolyte. XPS analyses demonstrate that the reduction products of ethylenesulfate, Li2SO3, and ROSO2Li are formed at the surface of graphite in the EC-based electrolyte, which is beneficial to lower the interfacial resistance as suggested by the EIS results. In addition, SEM images show a smoother and homogeneous surface film at the surface of graphite when ethylenesulfate is incorporated into the electrolyte. Consequently, the Li/graphite half cells cycled in EC-based electrolyte containing ethylenesulfate exhibit higher specific capacity and improved cycling capability than that without ethylenesulfate.[3]
Compatibility between graphite electrode and ethylenesulfate-containing electrolyte is investigated. It is found that ethylenesulfate can be used as an effective electrolyte additive to improve the cycling performance of the cell. The electrochemical tests show that the cell with electrolyte containing 1 wt% ethylenesulfate exhibits the highest capacity and the best cycle performance. The reason is that the electrolyte containing ethylenesulfate preferentially occupies the active sites of the graphite to form relatively thin and compact SEI film where only Li+ can pass through and result in lower interfacial impedance. Therefore, ethylenesulfate would be a promising film formation additive in lithium-ion battery.
References
[1] SOULBRAIN - KR2022/57819, 2022, A
[2] Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
[3] Li, X., Yin, Z., Li, X. et al. Ethylene sulfate as film formation additive to improve the compatibility of graphite electrode for lithium-ion battery. Ionics 20, 795–801 (2014).
See also
Lastest Price from ETHYLENESULFATE manufacturers
US $0.00/KG2025-04-21
- CAS:
- 1072-53-3
- Min. Order:
- 100KG
- Purity:
- 99.6%
- Supply Ability:
- 10mt
US $0.00/KG2025-04-21
- CAS:
- 1072-53-3
- Min. Order:
- 100KG
- Purity:
- 99.6%
- Supply Ability:
- 10mt