Ethyl 2-bromoisobutyrate: properties, applications and safety
General Description
Ethyl 2-bromoisobutyrate is an organic compound used in organic synthesis as a catalyst or starting material. The compound has a strong halogen group and can undergo multiple reactions, making it useful in various chemical reactions. One application of this compound is in the synthesis of cationic polymers for antibacterial peptide mimicry. These polymers showed promising antimicrobial activity against various strains of bacteria. However, Ethyl 2-bromoisobutyrate is a hazardous substance that requires careful handling to ensure safety. Appropriate personal protective equipment and ventilation should be used to prevent contact with the eyes, skin, or inhalation of vapors.

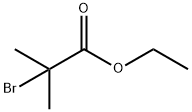
Figure 1. Ethyl 2-bromoisobutyrate
Properties
Ethyl 2-bromoisobutyrate is an organic compound with the chemical formula C6H11BrO2. It is a colorless liquid with a molecular weight of 167.06 g/mol and a density of 1.441 g/mL at 25°C. Its boiling point is between 136-138°C, and melting point between -61℃. The refractive index of this compound is 1.4538 at 20°C. This compound is a brominated alkyl ester and has a strong halogen group. It can be characterized by various spectroscopic methods including nuclear magnetic resonance (NMR). Although it is unstable at room temperature, it is relatively stable under low temperature and inert atmosphere. Ethyl 2-bromoisobutyrate is an important reagent in organic synthesis, often used as a catalyst or starting material. It can undergo multiple reactions including carbon-carbon bond formation, halogen exchange, and radical reactions. It is widely utilized in various chemical reactions such as atom transfer radical polymerization, Grignard reaction, Suzuki coupling reaction, etc. 1
Applications
Ethyl 2-bromoisobutyrate was used as an initiator in Cu(0)-mediated polymerization to synthesize a library of cationic polymers. These polymers were then assessed for their application as antibacterial peptide mimics. To begin, eight platform polymers with low degrees of polymerization (DP) were synthesized using (2-Boc-amino)ethyl acrylate as the monomer and either ethyl α-bromoisobutyrate or dodecyl 2-bromoisobutyrate as the initiator. This resulted in hydrocarbon chain termini of C2 or C12, respectively. A two-step modification strategy was employed to generate the final sixteen-member polymer library. The first step involved deprotection to reveal the primary amine cationic polymers, followed by guanylation. The antimicrobial activity of these cationic polymers was then assessed against various strains of bacteria, including Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Among the tested polymers, those with a short segment of guanidine units and a C12 hydrophobic terminus showed the broadest antimicrobial activity. Their minimum inhibitory concentration (MIC) values approached those of Gram-positive targeting antibacterial peptides such as daptomycin and vancomycin. To evaluate their cytotoxicity, the C12-terminated guanidine functional polymers were tested against human red blood cells. It was observed that as the degree of polymerization decreased, there was an increase in hemolysis. Cytotoxicity was also tested against HEK293 and HepG2 cells. The C12-terminated polymer with the lowest degree of polymerization exhibited minimal toxicity at the concentrations examined, except at the highest concentration. Further analysis indicated that membrane disruption was the likely mechanism of bacterial cell killing, as demonstrated by membrane permeability testing against E. coli. In summary, Ethyl 2-bromoisobutyrate was successfully used as an initiator in Cu(0)-mediated polymerization to synthesize a library of cationic polymers. These polymers showed promising antimicrobial activity, particularly those with a short guanidine segment and a C12 hydrophobic terminus. 2
Safety
Ethyl 2-bromoisobutyrate is a hazardous substance that requires careful handling to ensure safety. When working with this compound, it is important to wear appropriate personal protective equipment (PPE), including face protection such as goggles or a face shield, to prevent any contact with the eyes. Adequate ventilation should also be ensured to prevent inhalation of the vapors. It is crucial to avoid getting Ethyl 2-bromoisobutyrate on skin or clothing as it can cause severe skin irritation or chemical burns. In case of contact with skin or clothing, immediate removal and rinsing with plenty of water is necessary. If any symptoms of irritation occur, seek medical attention. As Ethyl 2-bromoisobutyrate is toxic if ingested, it is important to avoid ingestion at all costs. If accidentally ingested, do not induce vomiting and immediately seek medical attention. Inhalation of Ethyl 2-bromoisobutyrate vapors may result in headache, dizziness, and respiratory difficulties. It is important to avoid inhalation of the vapors by working with the compound in a properly ventilated area. Overall, proper handling and storage of Ethyl 2-bromoisobutyrate is crucial to ensure safety. 3
Reference
1. PubChem. COMPOUND SUMMARY: Ethyl 2-bromoisobutyrate. National Library of Medicine. 2005, CID: 11745.
2. Grace JL, Elliott AG, Huang JX, Schneider EK, Truong NP, Cooper MA, Li J, Davis TP, Quinn JF, Velkov T, Whittaker MR. Cationic Acrylate Oligomers Comprising Amino Acid Mimic Moieties Demonstrate Improved Antibacterial Killing Efficiency. J Mater Chem B. 2017 Jan 21;5(3):531-536.
3. SAFETY DATA SHEET: Ethyl 2-bromoisobutyrate. Thermo Fisher SCIENTIFIC, 2010.
You may like
Related articles And Qustion
See also
Lastest Price from Ethyl 2-bromoisobutyrate manufacturers

US $30.00-10.00/kg2025-03-07
- CAS:
- 600-00-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons

US $1.00/KG2024-10-11
- CAS:
- 600-00-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100000kg