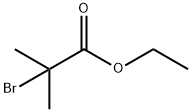
Ethyl 2-bromoisobutyrate: Overview, Other Properties and Synthesis
General Description
Ethyl 2-bromoisobutyrate is an important ester compound used in various chemical processes due to its unique properties. It can undergo hydrolysis to yield ethanol and 2-bromoisobutyric acid, making it a significant reaction in organic chemistry. Additionally, it plays a crucial role in the catalytic system for the controlled synthesis of polymers under visible light irradiation. The synthesis of Ethyl 2-bromoisobutyrate involves the esterification reaction between ethyl alcohol and 2-bromoisobutyric acid, catalyzed by sulfuric acid at elevated temperatures. This method is widely utilized on a commercial scale, making Ethyl 2-bromoisobutyrate an important intermediate in organic synthesis. Overall, Ethyl 2-bromoisobutyrate's properties and characteristics make it a valuable compound in various chemical processes and applications.

Figure 1. Ethyl 2-bromoisobutyrate
Overview
Ethyl 2-bromoisobutyrate is an ester compound with the molecular formula C6H11BrO2. It is formed from ethyl alcohol (ethanol) and 2-bromoisobutyric acid, resulting in the characteristic ester linkage -COO- in its structural formula: CH3 - CH(Br) - CH2 - COO - CH2 - CH3. This organic compound is a colorless to slightly yellow liquid with a distinct odor, and it exhibits solubility in organic solvents such as ether, chloroform, and acetone while being insoluble in water. In terms of chemical properties, Ethyl 2-bromoisobutyrate is relatively stable under normal conditions but can react with bases to produce the corresponding salt and alcohol. When heated, it undergoes ester hydrolysis, yielding ethanol and 2-bromoisobutyric acid. These properties make it a valuable compound in various chemical processes and applications. Overall, Ethyl 2-bromoisobutyrate's composition, physical appearance, and chemical reactivity position it as a significant player in organic chemistry, with potential uses in synthesis and other chemical reactions due to its unique characteristics and properties. 1
Other properties
Hydrolysis characteristics
Ethyl 2-bromoisobutyrate is a compound commonly used in organic synthesis, known for its unique hydrolysis characteristics. When ethyl 2-bromoisobutyrate is exposed to water or hydroxide ions, it undergoes hydrolysis through a nucleophilic substitution reaction. This process involves the breaking of the ester bond between the ethyl group and the bromoisobutyrate moiety, resulting in the formation of ethanol and 2-bromoisobutyric acid as the main products. The hydrolysis of ethyl 2-bromoisobutyrate is typically faster under basic conditions compared to neutral conditions due to the increased nucleophilicity of hydroxide ions. Additionally, the presence of electron-withdrawing groups on the bromoisobutyrate moiety can enhance the rate of hydrolysis by stabilizing the transition state. Overall, the hydrolysis of ethyl 2-bromoisobutyrate is a significant reaction in organic chemistry with practical applications in various synthetic pathways. 2
Catalytic characteristics
Ethyl 2-Bromoisobutyrate is a key component in the catalytic system, along with methylene blue, used in the polymerization of methyl methacrylate under visible light irradiation at room temperature. This catalytic system allows for the controlled synthesis of polymers, exhibiting significant features in terms of both monomer conversion and molecular weight characteristics. Under mild temperature conditions, the polymerization process operates according to the atom transfer mechanism, specifically the Metal Free Atom Transfer Radical Polymerization (ATRP). Even at low concentrations of photocatalyst, the polymerization can achieve high conversions, demonstrating the efficiency of the catalytic system. The presence of ethyl 2-Bromoisobutyrate in the system contributes to the controlled mode of polymerization, enabling precise regulation of the molecular weight characteristics of the resulting polymers. Together with methylene blue, it facilitates the synthesis of polymers with desired properties, highlighting its essential role in this photoirradiation-mediated polymerization process. 3
Synthesis
Ethyl 2-bromoisobutyrate is commonly synthesized through the esterification reaction between ethyl alcohol and 2-bromoisobutyric acid. This process is typically catalyzed by sulfuric acid and conducted at elevated temperatures. The synthesis begins with the combination of ethyl alcohol and 2-bromoisobutyric acid in the presence of sulfuric acid as a catalyst. The elevated temperature helps drive the esterification reaction forward, leading to the formation of Ethyl 2-bromoisobutyrate. During this reaction, a water molecule is eliminated, and the ester linkage -COO- is formed, resulting in the desired compound. This synthetic route is widely utilized for producing Ethyl 2-bromoisobutyrate on a commercial scale due to its efficiency and relatively straightforward procedure. The resulting compound can then be further utilized in various chemical processes and applications, making it an important intermediate in organic synthesis. In summary, the esterification reaction between ethyl alcohol and 2-bromoisobutyric acid, catalyzed by sulfuric acid at elevated temperatures, represents the primary method for synthesizing Ethyl 2-bromoisobutyrate, facilitating its production for diverse industrial and scientific purposes. 1
Reference
1. Ethyl 2-bromoisobutyrate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 11745.
2. WANG GL. HYDROLYSIS OF ETHYL-2-BROMOISOBUTYRATE ESTER IN AN ALKALINE SOLUTION. Chemical Engineering Communications. 2004, 191(1): 220-240.
3. LIZYAKINA OS, Vaganova LB. Features of Methyl Methacrylate Polymerization Mediated by Methylene Blue and Ethyl 2-Bromoisobutyrate under Photoirradiation Conditions. Polymer Science, Series B. 2023, 65(3): 270-283.
Related articles And Qustion
Lastest Price from Ethyl 2-bromoisobutyrate manufacturers

US $30.00-10.00/kg2025-03-07
- CAS:
- 600-00-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons

US $1.00/KG2024-10-11
- CAS:
- 600-00-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100000kg