Enzalutamide: An Androgen Receptor Antagonist for Metastatic Castration-Resistant Prostate Cancer
General Description
Enzalutamide, developed as a high-affinity and selective antagonist for the androgen receptor, has shown promising pharmacokinetic properties and clinical efficacy in treating metastatic castration-resistant prostate cancer. Its rapid absorption and prolonged half-life support once-daily dosing, with no need for dosage adjustment in patients with hepatic impairment. Phase I-II studies demonstrated its significant antitumor activity and a preferable safety profile, leading to the selection of a 160 mg daily dose for phase III trials. However, while enzalutamide extends median overall survival, its safety profile, including potential for severe adverse events and drug interactions, underscores the importance of careful patient monitoring and management to optimize treatment outcomes.

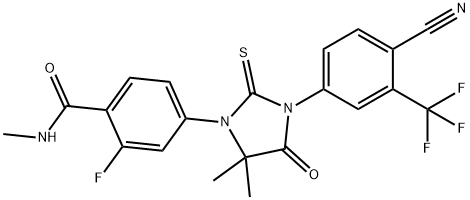
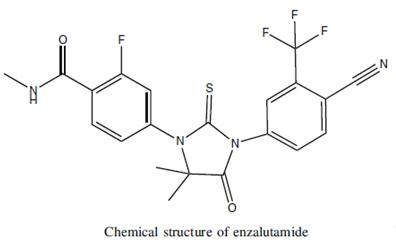
Figure 1. Enzalutamide
Pharmacokinetics
Enzalutamide, synthesized in 2006 as a high-affinity and selective antagonist for the androgen receptor (AR), exhibits notable pharmacokinetic properties. After oral administration, enzalutamide is rapidly absorbed, reaching peak serum concentrations within approximately one hour, with a range of 0.5 to 3 hours. This rapid absorption facilitates its once-daily dosing regimen. The drug demonstrates a half-life of 5.8 days, indicating its prolonged presence in the system, which supports steady-state serum concentrations being achieved within 28 days. At steady state, there is an 8.3-fold accumulation, showcasing its substantial systemic exposure. The pharmacokinetics of enzalutamide are dose-proportional across a range from 30 to 360 mg/day, with intersubject variability being less than 30%, highlighting its predictable behavior across different individuals. Enzalutamide primarily binds to albumin and undergoes metabolism in the liver, chiefly via cytochrome P450 enzymes CYP2C8 and CYP3A4. Despite its extensive hepatic metabolism, renal excretion plays a minimal role in its elimination. Importantly, no dosage adjustment is required for patients with varying degrees of hepatic impairment, encompassing mild, moderate, or severe conditions. This wide therapeutic window and manageable pharmacokinetic profile make enzalutamide a viable option for a broad patient population with metastatic castration-resistant prostate cancer. 1
Clinical efficacy
Enzalutamide, initially recognized as MDV3100, has demonstrated significant clinical efficacy in the treatment of castration-resistant prostate cancer (CRPC) through phase I-II studies. Designed to address the limitations of existing androgen receptor (AR) antagonists by offering higher binding affinity and pure antagonism, enzalutamide outperforms bicalutamide in pre-clinical models. Its first-in-human trial, conducted from July 2007 to December 2008, involved 140 men with progressive CRPC, showcasing its potential regardless of prior chemotherapy history. The trial aimed to establish safety, tolerability, and the maximum tolerated dose, alongside evaluating antitumoral activity through various metrics such as PSA changes, radiological assessments on metastases, time to progression, and circulating tumor cell count. Patients receiving doses from 30 to 600 mg/day exhibited antitumor activity across all levels. The maximum tolerated dose for prolonged treatment was identified at 240 mg, with the most common severe adverse events being dose-dependent fatigue and arthralgia. Notably, significant PSA level declines (≥50%) were observed in both chemotherapy-naïve patients (61%) and those with prior chemotherapy (52%). The study also reported responses in soft tissue metastases and stabilization of bone disease, indicating broad antitumoral effectiveness. Based on these outcomes, a daily dose of 160 mg was chosen for phase III trials due to its comparable activity to higher doses and reduced toxicity profile. This pivotal decision underscored enzalutamide's promise as a potent option for managing CRPC, highlighting its ability to significantly impact disease progression and improve patient outcomes. 1
Safety
Enzalutamide, a treatment option for men with metastatic castration-resistant prostate cancer, has been evaluated for safety in a phase III trial. Administered at 160 mg daily, it demonstrated significant efficacy in prolonging median overall survival to 18.4 months compared to 13.6 months with placebo. However, its safety profile requires careful consideration. Common adverse events associated with enzalutamide include asthenia or fatigue, back pain, arthralgia, hot flushes, peripheral oedema, musculoskeletal pain, and headache. Notably, enzalutamide was associated with higher frequencies of neutropenia and serious complications such as infections leading to death, falls or injuries from falls, and hallucinations compared to placebo. Additionally, there is a warning about seizures, with a small number of patients experiencing seizures during the trial. Cardiac disorders and hypertension were also reported. Given its extensive metabolism, primarily through cytochrome P450 (CYP) 2C8, and its role as a strong inducer of CYP3A4 and a moderate inducer of CYP2C9 and CYP2C19, enzalutamide may interact with various medications. Caution is advised in patients with moderate to severe hepatic or renal impairment. Despite its benefits in extending survival, the potential risks and drug interactions associated with enzalutamide necessitate careful patient selection and monitoring. 2
Reference
1. Altavilla A, Casadei C, Lolli C, et al. Enzalutamide for the treatment of nonmetastatic castration-resistant prostate cancer. Expert Opin Pharmacother. 2020;21(17):2091-2099.
2. Enzalutamide. Aust Prescr. 2015;38(1):28-29.
Related articles And Qustion
Lastest Price from Enzalutamide manufacturers

US $1.00-4.00/KG2025-05-15
- CAS:
- 915087-33-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG
US $0.00-0.00/KG2025-04-04
- CAS:
- 915087-33-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton