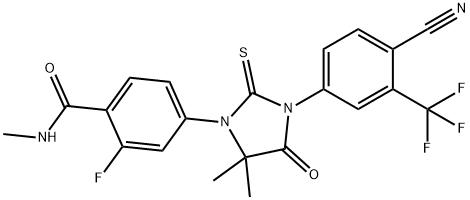
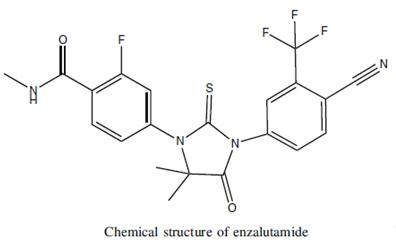
Enzalutamide: A Review on its Applications in Cancer
General Description
Enzalutamide is an androgen receptor (AR) inhibitor with a 5- to 8-fold greater affinity to bind AR than bicalutamide. Bicalutamide has partial agonistic activity, especially in the case of AR overexpression, and acts as an AR agonist. Antiandrogen withdrawal syndrome is the clinical manifestation of this agonistic affect. Enzalutamide was designed as a potent AR inhibitor without agonistic activity. Infact, enzalutamide is an AR signaling inhibitor. Unlike other antiandrogen therapies, enzalutamide also inhibits nuclear translocation of an activated AR to androgen response elements and coactivator recruitment. Enzalutamide also induces apoptosis while suppressing the growth of malignant prostate cells. These features differentiate enzalutamide from androgensynthesis inhibitors and other first-generation AR inhibitors. The efficacy of enzalutamide in prostate cancer has been proven in several phase II and phase III trials.1

Figure 1. Properties of Enzalutamide
Mechanism of action
Enzalutamide is a competitive androgen receptor (AR) inhibitor that has a threefold inhibition on the androgen signaling pathway without significant AR agonist activity. It inhibits androgen binding to its receptor, androgen receptor nuclear translocation, and subsequent interaction with chromosomal DNA to upregulate oncogenes. Enzalutamide binds to the AR with 5 to 8-fold greater affinity than first-generation antiandrogens such as bicalutamide and only 2-3 fold reduced affinity than the natural ligand dihydrotestosterone. Molecular docking showed that enzalutamide binds to the ligand binding domain of the AR distinctive from that of bicalutamide.2
Efficacy
The addition of enzalutamide to ADT in metastatic castration-resistant prostate cancer (CRPC) patients provided a significant PFS benefit compared with bicalutamide. In addition, the STRIVE study revealed that enzalutamide also prolongs PFS in patients without metastasis. The phase III PROSPER study also supports these results.Patients with nonmetastatic CRPC and a PSA doubling time ≤10 months and a PSA ≥2 ng/mL were randomized 2:1 into enzalutamide 160 mg and placebo groups while continuing ADT. The primary endpoint of the PROSPER trial was metastasisfree survival (MFS). The median MFS was significantly longer in the enzalutamide arm (36.6 months vs. 14.7 months; p<0.0001). Enzalutamide also significantly prolonged the time to PSA progression (37.2 months vs. 3.9 months; p<0.0001]).3
Enzalutamide has also been tested in hormone-naive prostatic cancer. A group of 67 hormone-naive prostate cancer patients (26 metastatic) were enrolled in an open-label phase II trial to assess the efficacy and safety of enzalutamide. In 62 patients, a PSA response (decline of 80% or more by week 25) was noted. At the second year, 67% of the patients were on treatment, and 73% of these had a PSA level of 0.1 ng/mL or less. Of the 26 patients with metastatic disease, 50% achieved a complete tumor response and 15% a partial response. The antitumor activity of enzalutamide is maintained at the third year. Enzalutamide is highly active in hormone-naive prostatic cancer, as well as in the castrate-resistant state.4
A single-arm phase II study evaluating the safety and antitumor activity of enzalutamide in AR-positive triple-negative breast cancer (TNBC) has been published. The primary endpoint was clinical benefit rate at week 16. The clinical benefit rate was 25% at week 16 in the intent-to-treat population (ITT) and 33% in patients whose tumor expressed 10% or more AR (evaluable group). The median PFS and OS was 2.9 months and 12.7 months, respectively, in the ITT group and 3.3 months and 17.6 months, respectively, in the evaluable group. The clinical benefit rate, disease-free survival and OS were numerically higher in the evaluable group. This study demonstrated the clinical activity of enzalutamide in advanced AR-positive TNBC.5
Pharmacokinetics
The median Tmax is 1 hour (0.5 to 3 hours) following a single 160 mg dose of capsules and 2 hours (0.5 to 6 hours) following a single 160 mg dose of tablets. Enzalutamide achieves steady-state by Day 28 and its AUC accumulates approximately 8.3-fold relative to a single dose. At steady-state, the mean (%CV) maximum concentration (Cmax) for enzalutamide and N-desmethyl enzalutamide is 16.6 µg/mL (23%) and 12.7 µg/mL (30%), respectively, and the mean (%CV) minimum concentrations (Cmin) are 11.4 µg/mL (26%) and 13.0 µg/mL (30%), respectively. Enzalutamide is 97% to 98% bound to plasma proteins, primarily albumin. N-desmethyl enzalutamide is 95% bound to plasma proteins. Enzalutamide is metabolized by CYP2C8 and CYP3A4. CYP2C8 is primarily responsible for the formation of the active metabolite (N-desmethyl enzalutamide). Carboxylesterase 1 metabolizes N-desmethyl enzalutamide and enzalutamide to the inactive carboxylic acid metabolite. The mean terminal half-life (t1/2) for enzalutamide in patients after a single oral dose is 5.8 days (range 2.8 to 10.2 days). Following a single 160 mg oral dose of enzalutamide in healthy volunteers, the mean terminal t1/2 for N-desmethyl enzalutamide is approximately 7.8 to 8.6 days.6
Adveser Effects
The most frequently reported adverse events with enzalutamide were fatigue, musculoskeletal pain (back pain), and hot flushes. Despite the fact that the duration of treatment in the enzalutamide arm was longer than that of the placebo or the standard treatment arm, treatment withdrawal rates due to adverse events were almost the same Grade 3 or higher hypertension was more often observed in the enzalutamide group than in the placebo group in the PREVAIL trial (13% vs. 4%), and more often than in the bicalutamide group in the STRIVE trial (10 patients vs. 3 patients) and the TERRAIN trial (13 patients vs. 8 patients). In a meta analysis, side affects of enzalutamide and other aleternative drug, abiraterone were assessed. All-grade (RR 1.28-95% CI 1.06-1.55) and grade ≥3 (RR 1.76-95% CI 1.12-2.75) cardiovascular event risk was higher with abirateron however there was no increase in risk of all-grade (RR 1.06-95% CI 0.67-1.65) or grade ≥3 (RR 0.81-95% CI 0.28-2.33) cardiovascular events in enzalutamide treated patients. Allgrade fatigue was significantly higher in the enzalutamide group (RR: 1.29; 95% CI: 1.15-1.44).7
Resistance to Enzalutamide
Acquired resistance to enzalutamide invariably emerges despite the impressive clinical activity in patients with prostate cancer. AR mutations, chiefly in the ligand-binding domain of the receptor, cause the development of antiandrogen resistance and progression of prostate cancer. Marcelli et al.[41] reported that 21% of patients with advanced prostate cancer had an AR mutation, while none of the patients with early stage disease had a mutation. A T877A mutation is frequently detected in CRPC. Antagonistic activity of enzalutamide is maintained in the T887A-mutated AR. Another important mutation in the ligand-binding domain of the androgen receptor is F876L. Enzalutamide-resistant, F876L mutations emerge when prostate cancer cells are cultured in the presence of enzalutamide. Enzalutamide demonstrates agonist activity at ARs containing this mutation.8
Synthesis
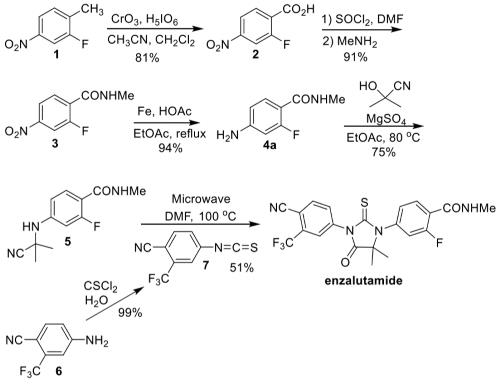
Figure 2. Synthesis of Enzalutamide
The first route to enzalutamide (Figure 2) was described by the groups of Sawyers and Jung at UCLA in a series of patent applications starting in 2006 and a journal publication in 2010. The synthesis started with 2-fluoro-4-nitrotoluene (1), which was oxidized to carboxylic acid 2 with chromium trioxide/ periodic acid in 81% yield. The acid was converted to the Nmethylamide 3 by activation with thionyl chloride followed by addition of methylamine. Reduction of the nitro group was accomplished with iron/HOAc to afford aniline 4a in 94% yield. Reaction of 4a with acetone cyanohydrin furnished nitrile 5 with an overall yield of 52% over the four steps. Separately, isothiocyanate 7 was prepared from aniline 6 with thiophosgene in water in a reported 99% yield. Coupling of nitrile 5 with isothiocyanate 7 was carried out under DMF at 100 °C for 11 h to afford enzalutamide in 51% yield after purification by chromatography.9
References
1. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787–90.
2. Schalken J, Fitzpatrick JM: Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016 Feb;117(2):215-25.
3. Hussain M, Fizazi K, Saad F, Rathenborg P, Shore ND, Demirhan E, et al. PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). Journal of Clinical Oncology 2018;36 Suppl 6:3.
4. Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Enzalutamide monotherapy in hormonenaive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol 2014;15:592–600.
5. Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol 2018;36:884–90.
6. FDA Approved Drug Products: Xtandi (enzalutamide) capsules/tablets for oral use 2022
7. Moreira RB, Debiasi M, Francini E, Nuzzo PV, Velasco G, Maluf FC, et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:84572–8.
8. Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer Discov 2013;3:1030–43.
9. Sawyers, C. L.; Jung, M. E.; Chen, C. D.; Ouk, S.; Welsbie, D.; Tran, C.; Wongvipat, J.; Yoo, D. Diarylhydantoin Compounds. PCT Int. Patent Appl. WO 2006/124118, November 23, 2006.
Related articles And Qustion
See also
Lastest Price from Enzalutamide manufacturers

US $1.00-4.00/KG2025-05-15
- CAS:
- 915087-33-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG
US $0.00-0.00/KG2025-04-04
- CAS:
- 915087-33-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton