Environmental Effects of Difluorochloromethane
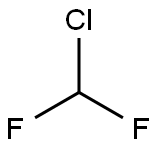
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). It is better known under its code names of HCFC-22, R-22, Genetron 22 or Freon 22, and is commonly used in air conditioning applications, such as residential split systems in the US, rooftop units and window air conditioners.
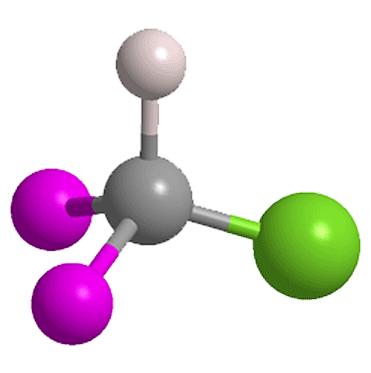
Chlorodifluoromethane was first used as an alternative to the highly ozone depleting CFC-11 and CFC-12 because of its relatively low ozone depletion potential of 0.055, among the lowest for chlorine-containing haloalkanes. However, even this lower ozone depletion potential is no longer considered acceptable it will be phased out soon under the Montreal Protocol, to be replaced by refrigerants with zero ozone depletion potential such as Propane (R-290), and other refrigerants (even though they don't have very similar properties): R-410A (an azeotropic mixture of difluoromethane and pentafluoroethane), R-502, R-134a (1,1,1,2-tetrafluroethane) and R-409A.
An additional environmental concern regarding Chlorodifluoromethane, as well as some of the proposed replacements, is their global warming potential. The global warming potential of Chlorodifluoromethane is 1700 (1700 times that of carbon dioxide). HFCs such as R-410A have high global warming potential, whereas that of propane (R-290) is only 3.
Environmental Effects
Chlorodifluoromethane is a colourless non-inflammable gas with a slightly sweet smell. It is hardly metabolized in man or animals; the only metabolites which have been described to date are CO2 and chloride ions. Chlorodifluoromethane is of very low toxicity to algae and aquatic invertebrates. For fish, no experimental data are available.
As it is a gas, any emitted Chlorodifluoromethane will quickly partition to the atmosphere, where it takes several years to photolyse. It will not partition significantly to soil or sediment due to its volatility and expected moderate adsorption. It is not expected to bioaccumulate in the food chain based on its volatility and low lipophilicity (log Kow= 1.13).
The acute and subacute toxicity of chlorodifluoromethane is relatively slight. The symptoms of intoxication comprise excitation and subsequent depression of the activity of the central nervous system. At very high concentrations chlorodifluoromethane causes cardiac sensitization to catecholamines.
![132-65-0 Dibenzo[b,d]thiopheneDibenzo[b,d]thiophene Applications](/NewsImg/2022-01-20/6377828939265470844310948.jpg)