Entecavir hydrate: Properties,Indications, Combination therapy and Possible complications
Entecavir hydrate properties
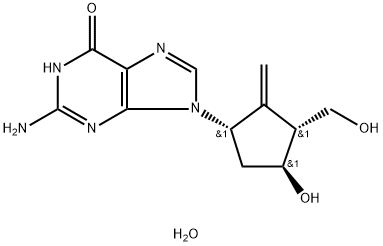
Entecavir hydrate (also kown as 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin6-one, monohydrate) is a guanosine nucleoside analogue, whose chemical formula is C12H15N5O3•H2O, and its molecular weight is 295.3.(Figure.1) Entecavir hydrate was originally developed for the treatment of herpes simplex virus infections,but its moderate activity led to its discontinuation for this indication. However, it was subsequently discovered that entecavir hydrate is a highly potent inhibitor of HBV, with low toxicity. Entecavir hydrate is converted intracellularly to its 5’-triphosphate form, which binds potently to the HBV polymerase; its in vitro potency is ~30-fold greater than that of lamivudine. By competing with the natural substrate deoxyguanosine triphosphate, entecavir triphosphate inhibits all the activities of HBV polymerase. It is only a weak inhibitor of cellular DNA polymerases. Entecavir hydrate showed good efficacy in the standard woodchuck and duck models of HBV infection. Importantly, after long-term therapy with entecavir hydrate in the woodchuck model,there was no emergence of drug resistance,and entecavir hydrate has also been shown to be active against lamivudine-resistant HBV in vitro.[1]

Indications
Entecavir is approved by the US FDA for the treatment of chronic HBV infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or aspartate aminotransferase (AST)) or histologically active disease.[1]
Combination therapy with entecavir hydrate plus tenofovir alafenamide fumarate
Long‐term administration of lamivudine (LAM) has been reported to lead to the emergence of drug‐resistant mutations, such as rtL180Mand rtM204V/I. Several studies have demonstrated that entecavir hydrate (ETV) shows mild cross‐resistance to LAM‐resistant mutations, such as rtL180M and rtM204V/I, whereas tenofovir (TFV) does not. However, it has been reported that the rtA194T mutation reduces TFV sensitivity by increasing the IC50 level in in vitro analyses. A recent study reported that the combination of quadruple mutations, namely rtS106C, rtH126Y, rtD134E, and rtL269I (CYEI) mutation, leads to a 15.3‐fold increase in the amount of TFV required to inhibit HBV at the IC50 level, and a 26.3‐fold increase at the IC90level in in vitro analyses. Thus, in patients currently receiving combination therapy with entecavir hydrate and tenofovir alafenamide fumarate (TAF) after the initial LAM administration, if LAM‐resistant mutations were to emerge, it is possible that antiviral efficacy would still be favorable even with TAF monotherapy, provided that there exist no HBV clones harboring the rtA194T or CYEI mutations.[2]
Patients with chronic hepatitis B virus (HBV) infection experiencing viral breakthrough (BTH) or partial response (PR) during lamivudine (LAM) or entecavir hydrate (ETV) administration often took ETV plus tenofovir alafenamide fumarate (TAF) due to the emergence of a drug-resistance mutation. However, in patients lacking drug-resistance mutation against TAF, sufficient antiviral effects may be achievable with TAF monotherapy. Yamada et al. assessed the drug-resistance profile through nucleotide sequences of HBV pregenome RNA, and subsequently changed to TAF monotherapy from entecavir hydrate plus TAF. This prospective study included 25 patients with serum HBV-DNA below 20 IU/mL under ETV plus TAF administration. Pregenome RNA nucleotide sequences of HBV in the sera were analyzed using direct sequencing and deep sequencing. Entecavir hydrate was discontinued in patients without rtA194T and rtS106C+rtH126Y+rtD134E+rtL269I quadruple mutations in direct sequencing. LAM-PR, LAM-BTH, ETV-PR, and ETV-BTH were observed in 1, 16, 7, and 1 patient(s), respectively. Pregenome RNA nucleotide sequences were analyzable in 20 patients. Among the 12 patients classified as LAM-BTH, six patients showed rtL180M+rtM204V/I in direct sequencing, and one patient showed minor clones containing rtL180M + rtM204V + A194T in deep sequencing at a frequency of 0.3%. In the six patients classified as ETV-PR, one patient harbored rtM204I. No clones showing rtS106C+rtH126Y+rtD134E+rtL269I quadruple mutation were detected in deep sequencing. Subsequently, entecavir hydrate was discontinued, and serum HBV-DNA remained undetectable up to 48 weeks in all patients. Patients receiving
entecavir hydrate plus TAF due to partial response or BTH during initial LAM or entecavir hydrate administration were able to safely transition to TAF monotherapy based on nucleotide sequences of HBV pregenome RNA in the sera.[2]
Entecavir hydrate-associated disease
Entecavir hydrate-associated thrombocytopenia
There are only a few reports about entecavir hydrate-associated thrombocytopenia, and it is considered as an immediate response and inappropriate to continue the treatment with other nucleoside analogues. Yu et al. had reported the third case, and this case was delayed response and they switched to treatment with tenofovir (TDF). There was a 66-year-old female who was infected with hepatitis B virus (HBV). Her platelet count decreased from 111*109/L to 3*109/L and was prone to gum bleeding and skin ecchymosis after she received entecavir hydrate treatment for 88 days. As a treatment option, entecavir hydrate was replaced by TDF immediately, frequent platelets transfusions and thrombopoietin were applied for several days, daily prednisone of 50 mg was concomitantly taken, and then platelet count improved after 10 days. She was diagnosed with entecavir hydrate-associated thrombocytopenia after analysis of the temporal relationship and exclusion of other causes of thrombocytopenia by blood and bone marrow examinations. Our case suggested that the platelet count should be monitored regularly in patients during entecavir hydrate treatment, and it may be a feasible option to choose TDF to maintain antiviral treatment when entecavir hydrate-associated thrombocytopenia occurs.[3]
Entecavir hydrate-associated myopathy
A 44-year-old man presented with a 3-month history of myalgia and progressive weakness. He had HBV infection and had received entecavir hydrate antiviral treatment for 5 years. Laboratory tests showed that serum creatine kinase levels were significantly elevated. Muscle histopathology showed abundant T-lymphocyte infiltration of muscle fibers, and HBV surface antigen and HBV core antigen were not present in muscle fibers. Entecavir-associated myopathy was subsequently diagnosed. The patient's symptoms eventually resolved, and serum CK levels decreased rapidly after he stopped receiving entecavir hydrate treatments. Conclusions: Patients who receive NA therapy should be closely monitored for myopathic side effects.[4]
Entecavir hydrate-induced Fanconi syndrome
Acquired Fanconi syndrome has been associated with the long-term ingestion of several nucleoside analogs used to treat chronic hepatitis B virus infection. However, the nucleoside analog entecavir hydrate has not been found to cause nephrotoxicity. Fujii et al. had reported a case of entecavir hydrate-induced Fanconi syndrome. The patient was a 73-year-old man admitted to our hospital because of renal dysfunction. He also presented with hyperaminoaciduria, renal diabetes, phosphaturia, hypophosphatemia, hypokalemia, hypouricemia, and hyperchloremic metabolic acidosis, supporting a diagnosis of Fanconi syndrome. In this case, the cause of Fanconi syndrome was most likely entecavir hydrate, which had been administered as needed depending on his renal function for 5 years. After drug discontinuation and replacement with tenofovir alafenamide fumarate therapy once a week, the patient's kidney function recovered and electrolyte anomalies partially improved. Researchers highlight the fact that entecavir may induce severe renal dysfunction, which can cause the development of Fanconi syndrome; therefore, close monitoring of proximal tubular function is recommended during entecavir hydrate therapy.[5]
References
1. Opio CK, Lee WM, Kirkpatrick P. Entecavir. Nat Rev Drug Discov. 2005;4(7):535-536. doi:10.1038/nrd1780
2. Yamada S, Uchida Y, Kouyama JI, et al. Switching from combination therapy with entecavir hydrate plus tenofovir alafenamide fumarate to tenofovir alafenamide fumarate monotherapy in patients with chronic hepatitis B based on nucleotide sequences of hepatitis B virus pregenome RNA. Hepatol Res. 2024;54(10):877-887. doi:10.1111/hepr.14036
3.Yu Y, Feng H. Entecavir-associated thrombocytopenia. Int J Immunopathol Pharmacol. 2021;35:20587384211059676. doi:10.1177/20587384211059676
4.Yuan K, Guochun W, Huang Z, Lin B, Zhou H, Lu X. Entecavir-associated myopathy: a case report and literature review. Muscle Nerve. 2014;49(4):610-614. doi:10.1002/mus.24118
5.Fujii T, Kawasoe K, Ohta A, Nitta K. A case of entecavir-induced Fanconi syndrome. CEN Case Rep. 2019;8(4):256-260. doi:10.1007/s13730-019-00404-5
You may like
See also
Lastest Price from Entecavir hydrate manufacturers
US $0.00/kg2025-11-27
- CAS:
- 209216-23-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/g2025-04-21
- CAS:
- 209216-23-9
- Min. Order:
- 1g
- Purity:
- 99.7%min
- Supply Ability:
- 1000g