Doxorubicin Hydrochloride Injection
Introduction
Doxorubicin Hydrochloride is a cytotoxic, anthracycline, topoisomerase II inhibitor indicated as a component of multi-agent adjuvant chemotherapy for the treatment of women with axillary lymph node involvement following resection of primary breast cancer. Doxorubicin hydrochloride is also indicated for treating certain metastatic diseases, leukemia and lymphoma. Doxorubicin hydrochloride is available in generic form.
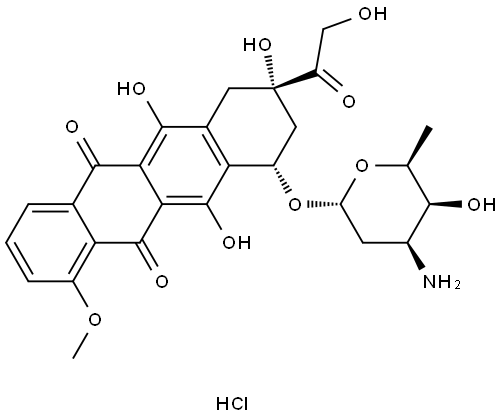
Doxorubicin is the active ingredient of doxorubicin hydrochloride. Doxorubicin is a cytotoxic anthracycline antibiotic isolated from Streptomyces peucetius var .caesius cultures. Doxorubicin consists of a naphthacenequinone nucleus linked through a glycosidic bond at ring atom 7 to an amino sugar, daunosamine. Chemically, doxorubicin hydrochloride is: 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L- lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl)-1- methoxy-, hydrochloride (8 S- cis)-. (8 S, 10 S)-10-[(3-Amino-2,3,6-trideoxy-α-L- lyxo-hexopyranosyl)-oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11- trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride.
Doxorubicin hydrochloride damages the cell's DNA and may kill cancer cells. It also blocks a certain enzyme needed for cell division and DNA repair. Doxorubicin hydrochloride is a type of anthracycline antibiotic and topoisomerase inhibitor.
Use
Doxorubicin Hydrochloride Injection, USP, has been used successfully to produce regression in disseminated neoplastic conditions such as acute lymphoblastic leukemia, acute myeloblastic leukemia, Wilms' tumour, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, ovarian carcinoma, transitional cell bladder carcinoma, thyroid carcinoma, gastric carcinoma, Hodgkin's disease, malignant lymphoma and bronchogenic carcinoma in which the small cell histologic type is the most responsive compared to other cell types.
Doxorubicin is also indicated for use as a component of adjuvant therapy in women with evidence of axillary lymph node involvement following resection of primary breast cancer.
Composition
Doxorubicin Hydrochloride Injection is a sterile, red-orange isotonic, preservative-free solution. Each milliliter of the injectable solution contains 2 mg doxorubicin hydrochloride with the following non-medical ingredients: sodium chloride, water for injection, hydrochloric acid and sodium hydroxide to adjust the pH to a target of 3.0. DOXORUBICIN HYDROCHLORIDE INJECTION is supplied in single-use snap-cap vials containing 10 mg (5 mL vials in boxes), 50 mg (25 mL vials in single boxes), and 200 mg (100 mL pharmacy bulk vials in single boxes). The rubber stoppers used in the vials are latex-free.
Incompatibility: Unless specific compatibility data are available, Doxorubicin Hydrochloride Injection should not be mixed with other drugs. Contact with alkaline solutions should be avoided since this can lead to hydrolysis of doxorubicin. Doxorubicin should not be mixed with heparin due to chemical incompatibility that may lead to precipitation. Precipitation also occurs with 5-fluorouracil.
You may like
Related articles And Qustion
See also
Lastest Price from Doxorubicin hydrochloride manufacturers

US $0.00/kg2025-05-23
- CAS:
- 25316-40-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $5.00-0.50/KG2025-05-07
- CAS:
- 25316-40-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS