Adverse reactions of doxorubicin hydrochloride
Introduction
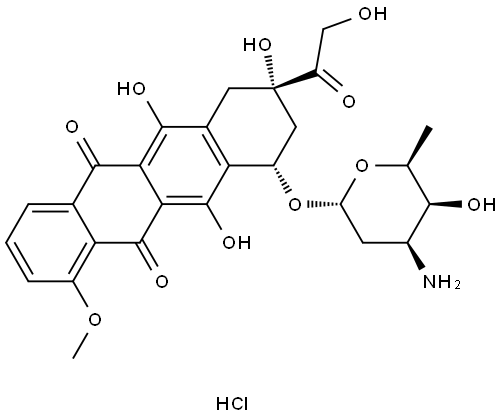
Doxorubicin hydrochloride belongs to antibiotics, with the molecular formula of C27H29NO11 · HCI[1]. It is an orange red loose block or powder, which is easily soluble in water, DMSO, tetrahydrofuran and alcohol, but insoluble in acetone, chloroform, benzene and ether. The solubility in water is 10mg / ml. It is an anti-tumor antibiotic. It can kill a variety of tumor cells by inhibiting the synthesis of genetic material nucleic acid of cancer cells and has a wide anti-tumor spectrum.

Picture 1 Doxorubicin hydrochloride powders
Doxorubicin hydrochloride is an anti-tumor antibiotic. By inhibiting the synthesis of nucleic acid of genetic material of cancer cells, this product has a wide anti-tumor spectrum and can kill a variety of tumor cells. Adriamycin hydrochloride for injection, also known as 14 hydroxydaunorubicin; 14 hydroxy orthomycin; Hydroxy positive dingmycin, adriamycin, adriamycin, hydroxydaunorubicin, hydroxyerythromycin, doxorubicin, adriamycin. Doxorubicin hydrochloride is an orange red loose block or powder, which is easily soluble in water, DMSO, tetrahydrofuran and alcohol. Insoluble in acetone, chloroform, benzene and ether. The solubility in water is 10mg / ml, which can be compatible with glucose and sodium chloride infusion; It can also be combined with some antitumor drugs.
Doxorubicin hydrochloride anti-tumor antibiotic has a wide anti-tumor spectrum and can kill a variety of tumor cells by inhibiting the synthesis of nucleic acid of genetic material of cancer cells. Adriamycin can produce a wide range of biochemical effects on the body. It has strong cytotoxicity. The main mechanism is that amygdala molecules are embedded into DNA and inhibit the synthesis of nucleic acid.
Absorption, distribution and excretion
Adriamycin disappeared quickly in plasma after intravenous injection. It is widely distributed in liver, spleen, kidney, lung and heart. This product is mainly metabolized in the liver, and about half of it is excreted from bile. Urinary excretion is about 4-5%, and liver dysfunction will lead to slower excretion. This product cannot pass through the blood-brain barrier. Medical / educational network.
Indication
This product is suitable for breast cancer, lung cancer, soft tissue tumors, osteosarcoma, bladder cancer, testicular cancer, thyroid cancer, neuroblastoma, Wilson cell carcinoma, malignant lymphoblastoma and acute leukemia. It can also be used in liver cancer, gastric cancer, ovarian cancer, cervical cancer, head and neck tumors, multiple myeloma, pancreatic cancer, esophageal cancer and endometrial tumor.
Adverse reactions and contraindications
Doxorubicin hydrochloride is a chemotherapeutic drug with strong cytotoxicity, so it does great damage to the body. Common adverse reactions are: inhibition of bone marrow hematopoietic function: manifested as thrombocytopenia and leucopenia. It has delayed cardiotoxicity, and heart failure can occur in severe cases. All patients had different degrees of hair loss, which could be recovered after drug withdrawal. Gastrointestinal reactions: nausea, vomiting, abdominal pain and ulceration of oral mucosa. Fever, hemorrhagic erythema and liver function damage can be seen in a few cases.
As a drug, doxorubicin hydrochloride is also contraindicated. It is forbidden for patients who have used other antitumor drugs or radiotherapy to cause bone marrow suppression. It is forbidden for patients with severe heart disease and pregnant women.
Adverse reaction
1. Inhibition of bone marrow hematopoietic function: manifested as thrombocytopenia and leucopenia.
2. It has delayed cardiotoxicity, and heart failure may occur in severe cases.
3. All patients have different degrees of hair loss, which can be recovered after drug withdrawal.
4. Gastrointestinal reaction: nausea, vomiting, abdominal pain and ulceration of oral mucosa.
5. Fever, hemorrhagic erythema and liver function damage can be seen in a few cases.
Attention
1. It must be used under the guidance of doctors. The changes of hemogram, liver function and ECG should be closely monitored during medication.
2. During intravenous infusion, the drug should be prevented from leaking out of the blood vessel to avoid tissue damage and necrosis.
3. This product cannot be combined with heparin to prevent precipitation.
4. The total accumulation (based on the total amount of medication) shall not exceed 450 ~ 550 mg per square meter (body surface area).
Reference
1 Li Guohua, Li Chenxi, Liu Xiaohang, etc Study on the adsorption performance of macroporous adsorption resin for adriamycin hydrochloride. Wang Fang, 2000
You may like
Related articles And Qustion
See also
Lastest Price from Doxorubicin hydrochloride manufacturers

US $0.00/kg2025-05-23
- CAS:
- 25316-40-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $5.00-0.50/KG2025-05-07
- CAS:
- 25316-40-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS