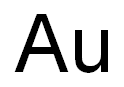Does pure gold oxidise?
Yes, Gold can lose electrons under special circumstances and can therefore be oxidised. However, gold is not oxidised by oxygen. As it is a transition metal in Group 11 of the periodic table, it is resistant to most oxidising agents and acids. Therefore, it is one of the least reactive precious metals and cannot combine effectively with oxygen, which means that it is resistant to corrosion and oxidation, and this only applies to pure or 24 karat solid gold. However, aqua regia (a 1:3 mixture of concentrated nitric and hydrochloric acid) will dissolve gold, as will an alkaline cyanide solution. The discolouration and corrosion of gold jewellery that we see in our daily lives is due to the fact that almost all gold manufactured items today are not 100% pure, but are usually made by alloying pure gold with harder metals. Over time, these harder metals will rust or lose their lustre, and the resulting gold alloy will also rust or lose its lustre.

You may like
Related articles And Qustion
See also
Lastest Price from GOLD manufacturers

US $1.10/g2025-04-17
- CAS:
- 7440-57-5
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min

US $10.00-15.00/g2023-03-11
- CAS:
- 7440-57-5
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 2546245