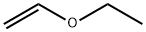Ethyl vinyl ether: Chemical properties and uses
Description
Ethyl vinyl ether (IUPAC name: Ethoxyethene), abbreviated as EVE, also known as ethoxy ethylene, vinyl ethyl ether, 1-ethoxy ethene, ethyl ethenyl ether, 1-ethoxy ethylene, and ethyloxyethene, is a derivative of ethanol and acetylene. The chemical formula of Ethyl vinyl ether is CH3CH2OCH=CH2. EVE can be prepared by reacting acetylene with absolute ethanol in the presence of an alkali catalyst.
Chemical properties
EVE is a colorless and transparent liquid with a boiling point of 35.7 °C. It is miscible with various organic solvents such as acetone, benzene, diethyl ether, heptane, methanol, and carbon tetrachloride. It is only slightly soluble in water. Under liquid–liquid equilibrium at 20 °C, the concentration of Ethyl vinyl ether in water is 0.9% by weight, while the amount of water dissolved in EVE is 0.2% by weight.

The vapor of Ethyl vinyl ether and air can form an explosive mixture. It is easy to burn and explode in case of open flame and high heat. The explosion limit (vol) ranges from 1.7% to 28%. Hence, the compound should be stored in a ventilated, low-temperature, and dry place, separately from oxidants and acids. It is chemically active and reacts violently with halogens and potent oxidizing agents. Self-polymerization can occur due to the action of light, heat, peroxide, and so on, and the polymerization reaction rapidly increases with the elevation of temperature. It is stable to alkalis but easily hydrolyzed to produce ethanol and acetaldehyde in the presence of inorganic acids. At low temperatures, Ethyl vinyl ether undergoes an addition reaction with hydrogen halide to give an α-halogenated hydrocarbon. Hydrogenation of EVE in the presence of Raney nickel catalyst generates diethyl ether.
Uses
Ethyl vinyl ether is a monomer for polymers, including homopolymers and copolymers. The homopolymer of EVE is a raw material for adhesives and paints. The Ethyl vinyl ether and maleic anhydride (MA) copolymer is widely used for pharmaceutical purposes as a thickening and suspending agent, denture adhesive, and adjuvant for transdermal patches.
It is also an intermediate for fine chemicals such as sulfadiazine and the disinfectant glutaraldehyde. Vinyl ether can paralyze the central nervous system, and its anesthetic effect is twice as strong as ether so that it can be used as an anesthetic and analgesic in medical treatment.
In addition, EVE is used in organic synthesis to protect the hydroxyl group, transvinylation, and cycloaddition reactions. EVE reacts with hydroxyl to form α-methoxyethyl ether, which is one of the most common and convenient ways to protect the hydroxyl group. Usually, the reaction requires a strong acid catalyst such as trifluoroacetic acid, p-toluenesulfonic acid, and pyridinium p-toluenesulfonate. α-Ethoxyethyl ether is easy to complete the deprotection reaction under acidic conditions. In the presence of catalysts (such as mercury acetate, phosphoric acid, and p-toluenesulfonic acid), EVE and allylic alcohols undergo transvinylation reactions to generate allyl vinyl ethers. This reaction is more significant because the products can undergo Claisen rearrangement to give unsaturated aldehydes. Moreover, Ethyl vinyl ether can also undergo a corresponding cyclization reaction in the presence of a Lewis acid catalyst.
You may like
See also
Lastest Price from Ethyl vinyl ether manufacturers

US $10.00/KG2025-04-21
- CAS:
- 109-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/Kg2025-04-21
- CAS:
- 109-92-2
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons