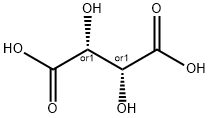
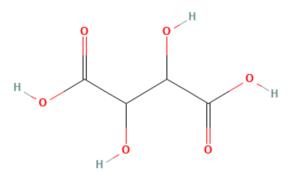
DL-Tartaric acid: Synthesis and Stereochemistry
Tartaric acid, also known as acidum tartaricum, 2,3-dihydroxybutanedioic acid (IUPAC nomenclature), is a white crystalline organic acid found naturally in many fruits with the formula C4H6O6. The molecule possesses two asymmetric carbon centers with two carboxylic acid groups and a dialcohol in the same molecule, resulting in four stereoisomers. DL-Tartaric acid (racemic mixture) is a synthetic equimolar combination of L(+) and D(-) forms. It is optically inactive due to internal compensation.

Synthesis of DL-Tartaric acid
3 mol of 1,4-dibromo-2,3-dimethoxy-succinaldehyde was added to the reaction vessel, and the mass fraction of 900 ml was 21% heptane solution, controlled stirring speed 190 rpm, raise the solution temperature to 46 ° C, add 8 mol aqueous solution, 8 mol dimethyl sulfoxide, add 8 mol mass fraction of 26% dioctyl sebacate solution in 40 min over 40 min. , continue to react for 80 minutes; B: Raise the temperature to 62 ° C, add 1.3L mass fraction of 15% potassium bromide solution, 5 mol of zinc acetate powder, continue The reaction was carried out for 3 h, the temperature was lowered to 16 ° C, and allowed to stand for 50 min. 700 ml of a 26% sodium nitrate solution was added, and the solution was layered, and the oil layer was separated and washed with a mass fraction of 45% 3-hexanol solution for 50 min. It was recrystallized from 67% cyclopentanoic acid solution and dehydrated with anhydrous magnesium sulfate dehydrating agent to obtain 442.35 g of finished DL-Tartaric acid, and the yield was 98.3%.[1]
Stereochemistry of DL-Tartaric acid
Naturally-occurring DL-Tartaric acid is chiral, meaning that it has molecules that are non-superimposable on their mirror-images. It is a useful raw material in organic chemistry for the synthesis of other chiral molecules. The naturally occurring form of the acid is L-(+)-tartaric acid or dextrotartaric acid. The mirror-image (enantiomeric) form, levotartaric acid or D-(−)-tartaric acid, and the achiral form, mesotartaric acid, can be made artificially. Note, that the dextro and levo prefixes are not related to the D/L configuration (which is derived from the reference DL-Tartaric acid), but to the orientation of the optical rotation, (+) = dextrorotatory, (−) = levorotatory. Sometimes, instead of capital letters, small italic d, l are used. They are abbreviations of dextro- and levo-, and nowadays should not be used. Levotartaric and dextrotartaric acid are enantiomers, mesotartaric acid is a diastereomer of both of them.
A rarely occurring optically inactive form of tartaric acid, DL-Tartaric acid is a 1:1 mixture of the levo and dextro forms. It is distinct from mesotartaric acid and was called racemic acid (from Latin racemus - "a bunch of grapes"). The word racemic later changed its meaning, becoming a general term for 1:1 enantiomeric mixtures - racemates. [2]
DL-Tartaric acid in wine
DL-Tartaric acid may be most immediately recognizable to wine drinkers as the source of "wine diamonds," the small potassium bitartrate crystals that sometimes form spontaneously on the cork. These DL-Tartaric acid are harmless, despite sometimes being mistaken for broken glass, and are prevented in many wines through cold stabilization. The tartrates that remain on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
However, DL-Tartaric acid plays an important role chemically, lowering the pH of fermenting "must" to a level where many undesirable spoilage bacteria cannot live, and acting as a preservative after fermentation. In the mouth, tartaric acid provides some of the tartness that is currently out of fashion in the wine world, although citric and malic acids also play a role. The modern practice of extended hang time, where grapes are allowed to sit on the vine nearly until they become raisins, can dramatically reduce the taste of tartaric acid in a wine, leaving it smoother but also potentially less compatible with food.[3]
References
[1] CHENGDU AOKATE TECHNOLOGY - CN108285413, 2018, A
[2] Brettle, R., Falshaw, C. P., & King, T. J. (1983). The stereochemistry of the tartaric acid chloralides. Journal of Chemical Research-S, (7), 164-165.
[3] Pilone, G. J. (1977). Determination of tartaric acid in wine. American Journal of Enology and Viticulture, 28(2), 104-107.
Related articles And Qustion
Lastest Price from DL-Tartaric acid manufacturers

US $1.00/g2025-04-21
- CAS:
- 133-37-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $100.00/KG2025-04-21
- CAS:
- 133-37-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON