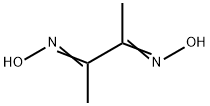
Dimethylglyoxime:Uses,Reactions,Preparation
Dimethylglyoxime is a white powder, soluble in methanol or sodium hydroxide solution. It is one of the first selective organic reagents applied in analytical chemistry.

Chemical reactions
Nickel cation reacts with dimethylglyoxime and forms an insoluble red precipitate of nickel dimethylglyoxime.
Ni2+ + 2C4H8N2O2 → Ni(C4H7N2O2)2↓(red precipitate) + 2H+
Dimethylglyoxime reacts with ferrous sulfate and ammonium hydroxide to form a complex compound of iron and ammonium sulfate and water is formed.
FeSO4 + 2NH4OH + 2C4H8N2O2 → Fe(C4H7N2O2)2 + (NH4)2SO4 + 2H2O
Uses
In analytical chemistry, Dimethylglyoxime is commonly used as a selective precipitating reagent, detecting reagent, and photometric reagent for nickel, palladium, platinum, and other metal ions;
It is useful for nickel release testing, as well as for jewelry and other skin-contact items;
Dimethylglyoxime works well as a nickel and palladium precipitant. Nickel precipitates as a vivid red voluminous complex from ammoniacal solution. As a result, the yellow compound is formed in dilute hydrochloric acid solutions containing white palladium.
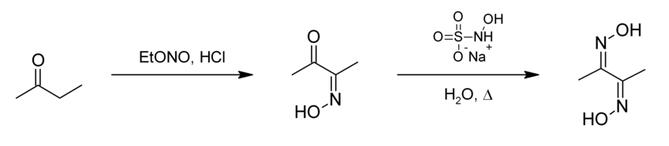
Preparation
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:
Related articles And Qustion
Lastest Price from Dimethylglyoxime manufacturers

US $5.00-1.00/kg2025-11-03
- CAS:
- 95-45-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 600tons

US $0.00/kg2025-09-09
- CAS:
- 95-45-4
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 2000kgs