Dibenzoyl-L-Tartaric Acid: Applications in Chiral Resolution and its Production by Continuous Method
General Description
Dibenzoyl-L-tartaric acid is essential in chiral resolution, particularly for separating enantiomers in racemic mixtures like albuterol through diastereomeric salt formation. The resolution process involves dissolving the racemic compound with Dibenzoyl-L-tartaric acid, resulting in a crystallizable diastereomeric salt. Recent advancements include its use in asymmetric photoreactions for deracemization. Additionally, Dibenzoyl-L-tartaric acid can be produced efficiently through a continuous method involving hydrolysis of diacyl tartaric anhydride, followed by controlled crystallization, ensuring high purity and yield suitable for both laboratory and industrial applications.

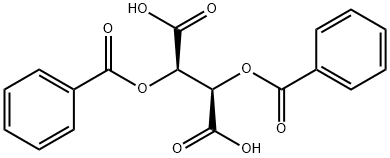
Figure 1. Dibenzoyl-L-tartaric acid
Applications in Chiral Resolution
Dibenzoyl-L-tartaric Acid in Chiral Resolution
Dibenzoyl-L-tartaric acid plays a crucial role in the resolution of chiral compounds, particularly in the separation of enantiomers of racemic mixtures. This compound, with its ability to form diastereomeric salts, is widely utilized to enhance the purity and yield of enantiomerically pure substances. In the context of chiral resolution, Dibenzoyl-L-tartaric acid is employed due to its effectiveness in forming stable crystalline complexes with racemic compounds, facilitating the isolation of individual enantiomers. For example, in the resolution of racemic albuterol, Dibenzoyl-L-tartaric acid is used to form a diastereomeric salt, which can then be separated and purified to yield optically active albuterol. This application highlights the importance of Dibenzoyl-L-tartaric acid in achieving high levels of chiral purity and resolving complex mixtures in pharmaceutical chemistry. 1
Experimental Application of Dibenzoyl-L-tartaric Acid
In practical scenarios, the use of Dibenzoyl-L-tartaric acid involves a series of well-defined procedures. For instance, when resolving racemic albuterol, a solution containing the racemic mixture is treated with Dibenzoyl-L-tartaric acid. The resulting mixture is heated and allowed to cool, leading to the formation of a diastereomeric salt. This salt is then filtered, washed, and recrystallized to obtain pure enantiomers. The efficiency of this method is demonstrated by the high diastereomeric excess and purity of the resolved product. Such methods underscore how Dibenzoyl-L-tartaric acid aids in achieving specific resolution goals, illustrating its utility in both laboratory and industrial settings for chiral separations.
Advanced Uses and Recent Developments
Recent developments have expanded the applications of Dibenzoyl-L-tartaric acid beyond traditional resolutions. In dynamic chiral salt formation, Dibenzoyl-L-tartaric acid has been employed in asymmetric photoreactions to achieve deracemization of quinolonecarboxamides. The process involves forming a crystalline salt with Dibenzoyl-L-tartaric acid, which then undergoes a photochemical reaction to produce enantiomerically pure products. This advanced technique demonstrates the versatility of Dibenzoyl-L-tartaric acid in modern chiral chemistry, showcasing its ability to maintain axial chirality over time and its effectiveness in high-yield asymmetric transformations. This innovative application highlights the evolving role of Dibenzoyl-L-tartaric acid in the field of stereochemistry and asymmetric synthesis. 2
Production by Continuous Method
Production of Dibenzoyl-L-tartaric Acid via Continuous Method
Dibenzoyl-L-tartaric acid can be efficiently produced using a continuous method that involves the hydrolysis of its corresponding diacyl tartaric anhydride. In this process, the diacyl tartaric anhydride is introduced into a hydrolysis reactor with water at a temperature range of 80-100°C. The hydrolysis reaction is maintained within this temperature range for 2-25 minutes, leading to the formation of a mixture consisting of dibenzoyl-L-tartaric acid monohydrate in an aqueous emulsion. This step ensures the transformation of the anhydride into the desired dibenzoyl-L-tartaric acid with high efficiency and precision. 3
Crystallization and Cooling of Dibenzoyl-L-tartaric Acid
After hydrolysis, the emulsion containing dibenzoyl-L-tartaric acid monohydrate is transferred to a pre-cooling reactor where it is cooled to a temperature of 65-85°C while maintaining its aqueous emulsion form. Following this, the emulsion is moved to a crystallizer, where the final crystallization of dibenzoyl-L-tartaric acid occurs at temperatures ranging from 10-55°C over a period of 5-20 minutes. This controlled crystallization process allows for the effective separation and formation of pure dibenzoyl-L-tartaric acid, ensuring high-quality production in a continuous manufacturing setting. 3
References:
[1] Q. ZHONG. A parallel approach to direct resolution of albuterol[J]. Chinese Science Bulletin, 2010, 11 1: 7933-7941. DOI:10.1007/S11434-010-3156-X.[2] FUMITOSHI YAGISHITA. Deracemization of Quinolonecarboxamides by Dynamic Crystalline Salt Formation and Asymmetric Photoreaction by Using the Frozen Chirality[J]. European Journal of Organic Chemistry, 2014, 2014 29: 6325-6564. DOI:10.1002/ejoc.201403056.
Lastest Price from Dibenzoyl-L-tartaric acid manufacturers

US $1.00/g2025-04-21
- CAS:
- 2743-38-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg

US $10.00/KG2025-04-21
- CAS:
- 2743-38-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt