Desonide: Topical Corticosteroid Efficacy and Pharmacokinetics
General Description
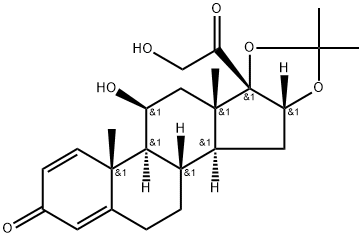
Desonide is a synthetic, nonfluorinated corticosteroid with the chemical formula C24H32O6, structurally similar to hydrocortisone. Its effectiveness is attributed to mechanisms such as vasoconstriction and anti-inflammatory properties, including stabilizing lysosomal membranes and inhibiting inflammatory mediators. Desonide also exhibits antiproliferative and antipruritic effects, making it useful in treating conditions like psoriasis and atopic dermatitis, particularly in children. Its pharmacokinetics are influenced by the formulation vehicle, skin condition, and barrier integrity, with enhanced absorption noted in inflamed or moisturized skin. Optimizing these factors can improve therapeutic outcomes, highlighting Desonide's versatility in dermatological applications.

Figure 1. Desonide
Topical Corticosteroid Efficacy
Chemical Structure and Composition
Desonide is a synthetic, nonfluorinated corticosteroid with a specific chemical structure that is pivotal in its pharmacological effects. Its chemical formula is C24H32O6, which reflects the precise arrangement of its atoms that contribute to its functionality. The structural backbone of Desonide is reminiscent of that of hydrocortisone, the first corticosteroid used topically, establishing a foundational chemistry that influences its efficacy. The compound's mode of action hinges on its interactions at the cellular level, where its lipophilic nature allows it to pass through cell membranes effectively. The ability of Desonide to form a complex with the cytoplasmic glucocorticoid receptor is essential for the subsequent biological processes that characterize its therapeutic applications. 1
Mechanisms of Action: Vasoconstriction and Anti-inflammatory Properties
The primary mechanisms of Desonide's action include vasoconstriction and anti-inflammatory effects, which are critical in its role as a topical corticosteroid. Vasoconstriction, a key indicator of corticosteroid potency, is achieved through the diminished sensitivity of vascular smooth muscle to agents like histamine. Additionally, Desonide stabilizes lysosomal membranes and inhibits the release of inflammatory mediators from mast cells. These actions are facilitated through both direct and indirect pathways; indirect effects involve the alteration of protein synthesis via protein–protein interactions with transcription factors, while direct mechanisms entail binding to cellular DNA, ultimately modifying gene transcription to regulate inflammation. The ability of Desonide to upregulate lipocortin-1 further elucidates its role in decreasing inflammatory mediators by inhibiting phospholipase A2, thereby limiting the production of prostaglandins and leukotrienes. 1
Clinical Applications: Antiproliferative and Antipruritic Effects
Several large studies have examined the efficacy and safety of desonide in the treatment of steroid-responsive dermatoses. In 1995, a randomized trial compared 0.05% desonide ointment to 1.0% hydrocortisone ointment in 113 children aged 10 months to 12 years with mild to moderate atopic dermatitis. Patients were randomized to receive treatment with desonide ointment or hydrocortisone ointment twice daily for 5 weeks, and 36 patients continued treatment for an additional 20 weeks. The physician's overall global assessment of improvement was the primary efficacy variable and included evaluation of erythema, lichenification, excoriations, oozing/crusting, induration/papules and pruritus.
Efficacy scores at all evaluations (weeks 1, 3 and 5, and months 2 to 6) showed greater efficacy and more rapid improvement with 0.05% desonide ointment than with 1.0% hydrocortisone ointment (69% desonide vs 41% hydrocortisone; p = 0.003). Neither treatment group showed signs of cutaneous atrophy, and any stinging or burning reported was slight, demonstrating equal topical safety profiles.1
Pharmacokinetics
Desonide is a topical corticosteroid with a specific pharmacokinetic profile influenced by various factors. The absorption of Desonide is dependent on the molecular structure of the drug, the formulation vehicle used, and the condition of the application site. Typically, Desonide is designed to penetrate the skin effectively, though its absorption can be altered based on the vehicle used. For instance, occlusive vehicles often enhance the penetration of Desonide compared to non-occlusive vehicles. Additionally, the skin's condition—whether inflamed, well-moisturized, or xerotic—significantly impacts how well Desonide is absorbed.
Factors Affecting Absorption
The absorption and activity of Desonide are also contingent on the integrity of the skin barrier. Areas with inflammation or compromised skin barriers generally show increased penetration of Desonide. Conversely, healthy, intact skin might exhibit lower absorption rates. Desonide's effectiveness is thus linked not only to its inherent properties but also to the state of the skin at the application site. Moisturization and the use of appropriate vehicles can enhance the therapeutic outcomes of Desonide by optimizing its delivery and absorption through the skin. 2
References:
[1] NR KAHANEK A H Cg Gelbard. Desonide: a review of formulations, efficacy and safety.[J]. ACS Applied Energy Materials, 2008. DOI:10.1517/13543784.17.7.1097.[2] YUANYUAN SUN. Evaluation of Bioequivalence and Pharmacokinetic Profiles for Topical Desonide Cream Using Chinese Skins.[J]. Skin Pharmacology and Physiology, 2024. DOI:10.1159/000540782.
Lastest Price from Desonide manufacturers

US $1000.00/KG2025-08-15
- CAS:
- 638-94-8
- Min. Order:
- 0.1KG
- Purity:
- 98%HPLC
- Supply Ability:
- 2tons

US $0.00/g2025-04-21
- CAS:
- 638-94-8
- Min. Order:
- 10g
- Purity:
- 97.0-103.0%,USP30
- Supply Ability:
- 50kg/month