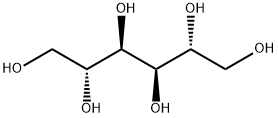
D-Mannitol, a naturally occurring alcohol
General description
D-Mannitol is a naturally occurring alcohol found in fruits and vegetables and used as an osmotic diuretic. Mannitol is freely filtered by the glomerulus and poorly reabsorbed from the renal tubule, thereby causing an increase in osmolarity of the glomerular filtrate. An increase in osmolarity limits tubular reabsorption of water and inhibits the renal tubular reabsorption of sodium, chloride, and other solutes, thereby promoting diuresis. In addition, mannitol elevates blood plasma osmolarity, resulting in enhanced flow of water from tissues into interstitial fluid and plasma.
Application and Pharmacology
1.Molecular dynamic simulations were performed in the solid and solution states of D-mannitol polymorphs to understand the spontaneous aqueous nucleation process microscopically. It is found that the molecular structure of D-mannitol in solution has no direct relationship with that in the solid state, whereas the form of dimers in the solution might relate to the final nucleation results at various supersaturation. Specifically, the H-dimers mostly appears in the solution at supersaturation of 1.27 where the beta form nucleated according to the experiments. Meanwhile, the I-dimers are found when the supersaturation is increased to 1.90, where the delta polymorph was obtained. While at the highest supersaturation of 2.92, there is no H-dimers or I-dimers in the solution, and 2 different T-dimers are observed. Moreover, as the interaction energy of H-dimer is the largest, followed by T-dimer, and the smallest is I-dimer, it is not difficult tofind that the order of stability of the dimers is consistent with that of the finally formed crystal forms.19So we have reason to believe that the H-dimer might be the reason for the nucleation of beta D-mannitol.
Figure 1 Intermolecular connection between a central object (red) and other adjacent D-mannitol molecules (yellow: 3 hydrogen bonds formed with the central object, green: 2 hydrogen bonds formed with the central object, gray: 1 hydrogen bond formed with the central object). (a) alpha; (b) beta; (c) delta[1]
2.Mannitol is an osmotic diuretic that is metabolically inert in humans and occurs naturally, as a sugar or sugar alcohol, in fruits and vegetables. Mannitol elevates blood plasma osmolality, resulting in enhanced flow of water from tissues, including the brain and cerebrospinal fluid, into interstitial fluid and plasma. As a result, cerebral edema, elevated intracranial pressure, and cerebrospinal fluid volume and pressure may be reduced. Mannitol may also be used for the promotion of diuresis before irreversible renal failure becomes established; the promotion of urinary excretion of toxic substances; as an Antiglaucoma agent; and as a renal function diagnostic aid. On October 30, 2020, mannitol was approved by the FDA as add-on maintenance therapy for the control of pulmonary symptoms associated with cystic fibrosis in adult patients and is currently marketed for this indication under the name D-mannitol appears as odorless white crystalline powder or free-flowing granules. Sweet taste.
Synthesis
1.D-mannitol was prepared from Laminaria japonica Aresch via 95% ethanol extraction, vacuum concentration, repeated column chromatography separation, recrystallization of petroleum ether-ethyl acetate, and residual solvent removal. Its chemical structure was identified by elemental analysis, optical activity analysis, infrared spectrum, mass spectrum, and nuclear magnetic resonance methods. Its homogeneity, stability, and cooperative certification by 8 laboratories were systematically checked by high performance liquid chromatography with an evaporative light-scattering detector[2].
2.Archaeal hyperthermostable mannitol dehydrogenases: A promising industrial enzymes for D-mannitol synthesis:. In particular, metabolic engineering for D-mannitol considers one of the most promising approaches for D-mannitol production on the industrial scale. To date, the chemical process is not ideal for large-scale production because of its multistep mechanism involving hydrogenation and high cost. In this review, we highlight and present a comparative evaluation of the biochemical parameters that affecting D-mannitol synthesis from Thermotoga neapolitana and Thermotoga maritimamannitol dehydrogenase (MtDH) as a potential contribution for D-mannitol bio-synthesis. These species were selected because purified mannitol dehydrogenases from both strains have been reported to produce D-mannitol with no sorbitol formation under temperatures (90–120 °C)[3].
Figure 2 D-mannitol production via hyperthermophilic MtDH. (1)D-mannitol production pathway using Thermotoga maritima MtDH. (2) D-mannitol production pathway using Thermotoga neapolitana MtDH.
References
1.Koko M. Y. F., Mu W. & Hassanin H. A. M. et al., "Archaeal hyperthermostable mannitol dehydrogenases: A promising industrial enzymes for d-mannitol synthesis," Food Research International, Vol.137(2020), p.109638.
2.Jin Wenhui, Chen Weizhu, Zhang Yiping, etc.: development of D-Mannitol standard sample, Zhongnan pharmacy, No. 12, 2021, pp. 2639-2644.
3.Su W., Zhang Y. & Liu J. et al., "Molecular Dynamic Simulation of D-Mannitol Polymorphs in Solid State and in Solution Relating With Spontaneous Nucleation," Journal of Pharmaceutical Sciences, Vol.109, No.4(2020), pp.1537-1546.
You may like
Related articles And Qustion
See also
Lastest Price from D-Mannitol manufacturers
US $0.00/kg2025-08-07
- CAS:
- 69-65-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $0.00/kg2025-04-27
- CAS:
- 69-65-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg