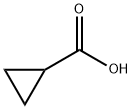
Cyclopropanecarboxylic Acid: Solubility, Microwave Spectrum, Metabolism, and Ester Prodrugs
Cyclopropanecarboxylic acid is a monocarboxylic acid and a member of cyclopropanes. It is a conjugate acid of a cyclopropanecarboxylate. Polar Solvents: Cyclopropanecarboxylic acid is soluble in polar solvents such as water and ethanol. The presence of the carboxyl group (-COOH) allows it to form hydrogen bonds with water molecules, enhancing its solubility. Non-polar Solvents: Its solubility in non-polar solvents is limited. The cyclopropane ring's hydrophobic nature contributes to its lower solubility in oils or organic solvents that do not exhibit polar characteristics. Concentration Effects: Higher concentrations may increase the solubility somewhat due to the presence of more functional groups available for interaction with solvent molecules. In general, cyclopropanecarboxylic acid demonstrates that structure significantly influences solubility, particularly due to the functional group present. As one might say, "the devil is in the details," and understanding these details can lead to a greater appreciation of how compounds behave in various environments. As chemists continue to explore cyclopropanecarboxylic acid, the compound demonstrates how simple structures can lead to complex behaviors and applications in the world of chemistry. Its manipulation in synthetic pathways showcases the creativity and innovation inherent in chemical research.

Microwave Spectrum of Cyclopropanecarboxylic Acid
Cyclopropane and its derivatives are reactive, versatile compounds that can be found throughout nature. They are important throughout biology and can be synthesized and utilized for many important reactions. Structural information on these types of molecules is important to obtain to understand the chemistry and reactivity behind these enzymatic processes in biology or reactions performed in the lab. Infrared, Raman, microwave, and X-ray crystal structure studies have been reported previously for cyclopropanecarboxylic acid (CPCA). To obtain a more accurate gas-phase structure, transitions from 13C singly substituted isotopologues were needed to continue from the previous work. This work extends the microwave spectroscopic work to include these 13C measurements and allows us to obtain a best-fit gas-phase structure of Cyclopropanecarboxylic acid. The Gaussian program with density functional theory and MP2 methods have been reasonably well tested and successful for ground-state singlet molecules but should really be tested further for excited states. This work provides an example test. Spectra for a higher-energy conformer for Cyclopropanecarboxylic acid were accurately measured using a high-resolution pulsed-beam Fourier transform microwave spectrometer. The experimental best-fit rotational constants agreed within 1% of the rotational constants obtained from our B3LYP calculations and within <1% with previous MP2 calculations. [1]
After searching for transitions corresponding to the higher-energy conformer with calculated rotational constants for the geometry shown of Cyclopropanecarboxylic acid, 10 weak rotational transitions were measured and definitively assigned to be from this conformer. Comparing the measured rotational constants obtained with results from high-level Gaussian calculations allowed us to clearly associate this structure with the cis conformation. This indicates that the abundance of the excited-state molecules is significantly less than predicted from the relative energies. The calculated abundances for a room-temperature sample are 85% and 15%. Our calculations predicted 83% (lower state) and 17% (higher-energy state). The present observations indicate relative abundances of 99% (lower state) and 1% (higher-energy state for our experimental conditions (cooled supersonic beam with neon carrier gas). To obtain an accurate gas-phase structure of the low-energy conformer of Cyclopropanecarboxylic acid, transitions from 13C single substituted isotopologues were needed to continue from the previous work. Measurements were extended for the low-energy conformer of CPCA to include single 13C substitutions at all C positions. A Kraitchman analysis was performed on this CPCA conformer, and these Kraitchman coordinates, along with the best-fit structure coordinates.
Metabolism of Cyclopropanecarboxylic Acid
Cells of the fungus Fusarium oxysporum Schlectendahl grown on cyclopropanecarboxylic acid as sole carbon source convert cyclopropanecarboxylic acid to y-hydroxybutyric acid through the intermediate cyclopropanecarboxylate-X. This derivative can be converted to y-hydroxybutyric acid by cell-free ex- tracts of the fungus. Preliminary investigation of the chemical properties of cyclopropanecarboxylate-X revealed that mild acidic or basic hydrolysis resulted in release of the intact cyclo- propylcarbonyl moiety. Ion exchange chromatography indicated that the derivative contained a positive charge. In this study the partial purification of cyclopropanecarboxylate-X will be described and evidence will be presented that indicates that this derivative is cyclopropanecarboxylate carnitine. Inclusion of dl-carnitine in the incubation mixture of whole cells of the fungus with [1-14C]cyclopropanecarboxylic acid resulted in an increase in the amount of [1-14C]cyclopropanecarboxylate-X synthesized. Authentic [1-14C]cyclopropanecarboxylate dl-carnitine has the same retention volume on Sephadex G-10 and LH-20 chromatography as does cyclopropanecarboxylate-X. Both compounds behave similarly on thin layer chromatography in several solvent systems.[2]
The organism F. oxysporum can degrade the cyclopropane ring of cyclopropanecarboxylic acid in order to fulfill its metabolic requirements. The sequence of reactions involved in the degradation of cyclopropanecarboxylic acid involve an “activation” of cyclopropanecarboxylic acid to cyclopropanecarboxylate-X. Increased amounts of cyclopropanecarboxylate-X were obtained by utilizing y-hydroxybutyrate instead of cyclopropane- carboxylic acid as sole carbon source for growth of F. oxysporum. Cyclopropanecarboxylate-X was obtained in radiopure form, but was not chemically pure as determined by mass spectral analysis. Enzymatic assay of a hydrolyzed sample of the compound indicated the presence of L-carnitine in the products of hydrolysis, but the molar ratio of L-carnitine to cyclopropanecarboxylate was less than unity. Investigation of the chemical properties of radiopure cyclopropanecarboxylic acid-X further indicated X might be carnitine.
Cyclopropanecarboxylic Acid Esters as Potential Prodrugs
Prodrugs with ester functionalities have the significant potential to increase the oral availability of otherwise potent orally unavailable therapeutics. We have found that cyclopropanecarboxylic acid esters can be used as prodrugs that can provide increased stability in the acidic environment of the stomach and the alkaline conditions present in the intestine. Increased stability should result in better absorption of the intact prodrug into the plasma. Esters of cyclopropanecarboxylic acid demonstrate a substantial increase in stability under both acid- and base-catalyzed hydrolytic conditions. Comparison of the stability of valacyclovir with the cyclopropane analogue shows that at 40 °C and pH 6 the half-life of 14 is >300 h while the value for 13 is 69.7 h. CBS-QB3 calculations on isodesmic reactions for transfer of groups from an alkane to an ester show that a cyclopropyl group provides hyperconjugative stabilization.[3]
References
[1]Pejlovas, A. M., Lin, W., & Kukolich, S. G. (2015). Microwave spectrum for a second higher energy conformer of cyclopropanecarboxylic acid and determination of the gas phase structure of the ground state. The Journal of Physical Chemistry A, 119(39), 10016-10021. https://doi.org/10.1021/acs.jpca.5b06733
[2]Guilbert, C C, and A E Chung. “Metabolism of cyclopropanecarboxylic acid. A new role for carnitine.” The Journal of biological chemistry vol. 249,4 (1974): 1026-30.
[3]Bender, David M et al. “Cyclopropanecarboxylic acid esters as potential prodrugs with enhanced hydrolytic stability.” Organic letters vol. 10,3 (2008): 509-11. doi:10.1021/ol702892e
You may like
See also
Lastest Price from Cyclopropanecarboxylic acid manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 1759-53-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00/KG2025-04-21
- CAS:
- 1759-53-1
- Min. Order:
- 1KG
- Purity:
- 97%
- Supply Ability:
- 500kgs/month