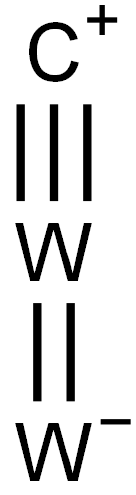Crystal structure and Uses of ?Tungsten carbide
Tungsten carbide has the chemical symbol WC. In its natural state, it is a fine grey powder, which can either be sintered (compacted using heat or pressure) onto a surface, or cemented (bound with a metal to form a solid).
Crystal structure
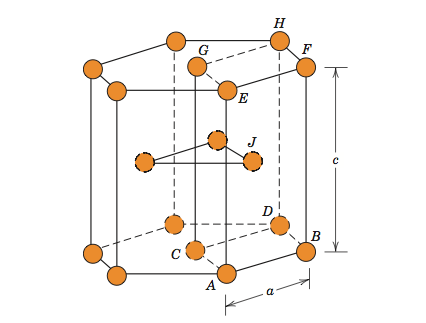
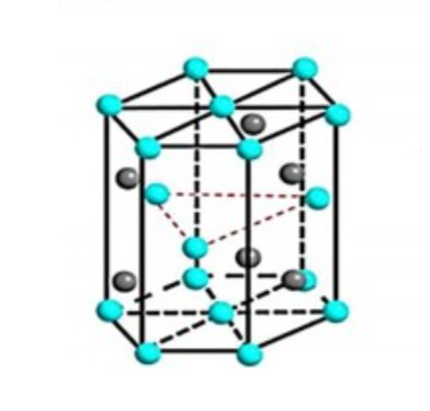
Tungsten carbide has a hexagonal form, α-WC (hP2, space group P6m2, No. 187). The hexagonal form can be visualized as made up of a simple hexagonal lattice of metal atoms of layers lying directly over one another (i.e. not close-packed), with carbon atoms filling half the interstices giving both tungsten and carbon a regular trigonal prismatic, 6 coordination.
Uses
Tungsten carbide is a compound of tungsten and carbon, renowned in the industry for its superior durability and high melting point (2,870℃). It is widely used in applications that require superior wear or impact resistance, such as abrasives, cutters, dies, and punches.
Synthesis
Tungsten carbide is prepared by reaction of tungsten metal and carbon at 1,400–2,000℃. Other methods include a lower temperature fluid bed process that reacts either tungsten metal or blue WO3 with CO/CO2 mixture and H2 between 900 and 1,200 ℃.
Health hazards
The primary health risks associated with tungsten carbide relate to inhalation of dust, leading to silicosis-like pulmonary fibrosis. Cobalt-cemented tungsten carbide is also anticipated to be a human carcinogen by the American National Toxicology Program.