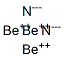Crystal structure and Uses of Beryllium nitride
Beryllium nitride is a compound of beryllium and nitrogen, prepared by heating beryllium metal powder with dry nitrogen at high temperatures (1100-1500 ℃). It is relatively reactive, rapidly oxidized in air at 600 ℃, and decomposed by water.
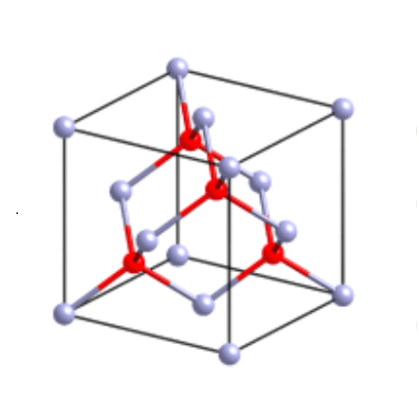
Crystal structure
Beryllium nitride is a gray cubic crystal; hard and refractory.
Preparation
Beryllium nitride is prepared by heating beryllium metal powder with dry nitrogen in an oxygen-free atmosphere in temperatures between 700 and 1400 °C.
3Be + N2 → Be3N2
Uses
Beryllium nitride is used in refractory ceramics as well as in nuclear reactors and to produce radioactive carbon-14 for tracer applications.
Beryllium Nitride (Be3N2) powder finds applications in semiconductors, high thermal conductivity materials, nuclear industry, optical coatings, batteries, and materials research. Caution is needed due to its potential hazards.