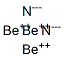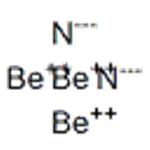Description
Beryllium nitride has the molecular formula of Be3N2 and the molecular weight of 55.0652 g/mol. It can be prepared from the elements at high temperature (1100– 1500 °C). Be3N2 decomposes in vacuum into beryllium and nitrogen:
3Be +N2 → Be3N2
It is a white to yellow powder with a density of 2.71 g/cm3. Its melting point is 2208 °C and a boiling point of 2240 °C. Its CAS number is 1304-54-7. It is soluble in water and is readily hydrolyzed forming beryllium hydroxide and ammonia:
Be3N2 + 3H203→Be(OH)2 + 2NH3
It has two polymorphic forms: cubic a-Be3N2 with a defect anti-bixbyite structure (a = 8.1452 ), and hexagonal β-Be3N2.
Chemical Properties
triberyllium nitride is hard, refractory, white crystals. Readily attached by strong alkali solutions, liberating ammonia.
Physical properties
Hard white or grayish crystal.
Oxidizes in air above 600°C.
Slowly decomposes in water,
quickly in acids and alkalis with
evolution of NH
3.
Physical properties
Gray cubic crystal; hard and refractory; density 2.71 g/cm
3; melts at 2,200°C; decomposes in acid or alkali; slowly reacts with water.
Uses
triberyllium nitride productions of the radioactive carbon isotope1 4C for tracer uses.
Preparation
Beryllium nitride may be prepared by heating beryllium metal powder with dry nitrogen in an oxygen-free atmosphere above 700°C.
3Be + N
2→Be
3N
2
Hazard
Chronic inhalation of the powder can cause cancer and adverse reproductive effects.