Chlorothalonil Pesticides: Toxicity and Detection
Basic Introduction
Chlorothalonil is a broad-spectrum organochloride pesticide. [1] It is primarily used as a fungicide, bactericide, and nematicide, and has been reported to be effective on a wide range of vegetables and fruit crops. It is also used as a bactericide, nematocide, and mildew-preventing agent in paints. The primary routes of exposure to chlorothalonil are ingestion, inhalation, dermal, and ocular. Occupational exposure to chlorothalonil may occur through inhalation of dusts or dermal contact with this compound at workplaces where it is produced or used as a pesticide. The greatest potential for dermal and inhalation exposure to chlorothalonil is expected for pesticide applicators and farm workers who have frequent contact with products containing this compound. Monitoring data indicate that the general population may be exposed to chlorothalonil via inhalation of ambient air and ingestion of food. Chlorothalonil gets rapidly absorbed when ingested and inhaled. Liver is the primary site of metabolism of chlorothalonil via conjugation with glutathione. It is a hepato-, nephro-, neuro-, and reproductive toxin with carcinogenic potential. It is a well-known skin and eye irritant that is reported to cause severe hypersensitivity reaction even in the absence of direct skin contact, owing to its high volatility. Typical hypersensitivity reactions following chlorothalonil exposure include erythema, edema, eczema, and pruritus. Chlorothalonil is moderately persistent in soil, and its primary breakdown product in soil is 4-hydroxy-2,5,6-trichloroisophthalonitrile. Chlorothalonil is considered Group B2, probable human carcinogen. In case of overexposure, gastrointestinal decontamination and administration of activated charcoal along with cathartic sorbitol should be considered.
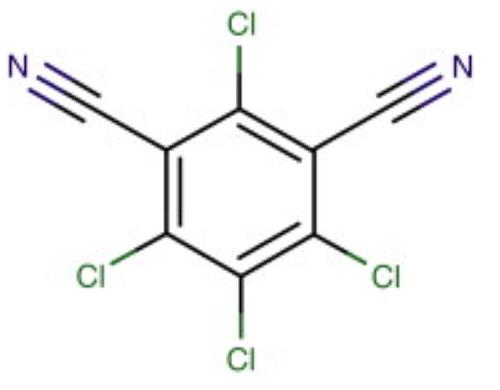
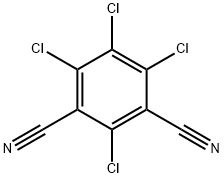
Chlorothalonil is an aromatic halogen compound that appears as a grayish to colorless crystalline solid that is odorless or has a slightly pungent odor. It has a molecular weight of 265.92, water solubility of 0.6 mg l-1 at room temperature, a melting point of 250–251 °C, and a boiling point of 350 °C. Chlorothalonil is only slightly soluble in acetone, dimethyl sulfoxide, cyclohexane, and xylene. It is noncorrosive and stable in moderately alkaline or acidic aqueous solutions. At high temperatures, chlorothalonil decomposes to emit hydrochloric acid. Some popular trade names for chlorothalonil include Bravo, Daconil 2787, Echo, Exotherm Termil, Nopcocide, Repulse, and Tuffcide. This compound can be found in formulations with many other pesticide compounds. Chlorothalonil is an important broad-spectrum, nonsystemic, organochlorine fungicide that has been widely used for more than 30 years as an effective disease management tool for potatoes, peanuts, turf, and vegetable and fruit crops. It is also used to control fruit rots in cranberry bogs and is used in paints. Chlorothalonil is classified as a general use pesticide by the US Environmental Protection Agency (EPA). Typical chlorothalonil application rates are 1 kg ha-1with four to nine applications per growing season. Chlorothalonil can enter surface waters through rainfall runoff, spray drift, or atmospheric deposition, subsequently having an impact on the aquatic biota. Most common routes of exposure to chlorothalonil are via the skin and eyes; it may also be ingested or inhaled.
Environmental Fate and Behavior
Chlorothalonil's production and use as a broad-spectrum, nonsystemic, protectant pesticide results in its direct release to the environment.[2] Its uses as a wood protectant, antimold and antimildew agent, bactericide, microbiocide, algaecide, insecticide, and acaricide are additional routes of release. If released to air, chlorothalonil will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase chlorothalonil will be degraded slowly in the atmosphere by reaction with photochemically produced hydroxyl radicals (reaction half-life ∼7 years). Direct photolysis may also occur. Chlorothalonil is removed from the atmosphere by wet and dry deposition. If released to soil, chlorothalonil is expected to have low mobility or be immobile, based on Koc values in the range of 900–7000 measured in four soils. Volatilization from moist or dry soil surfaces is not expected to be important based on a Henry's Law constant of 2.5 × 10−7 atm-cu m mol-1. Aerobic biodegradation half-lives of chlorothalonil in four different soils ranged from 10 to 40 days. If released into water, chlorothalonil is expected to adsorb to suspended solids and sediment in the water column.

Biodegradation is expected to be an important fate process given aerobic aquatic degradation half-lives of 8.1 and 8.8 days measured in marine water, and anaerobic degradation half-lives ranging from 5 to 15 days measured in flooded soils. Hydrolysis does not occur under acidic conditions or at pH 7; however, a hydrolysis half-life of 38.1 days was observed for chlorothalonil at pH 9. An aqueous photolysis half-life of 65 days was measured for chlorothalonil, suggesting photolysis in sunlit surface waters is possible. Bioconcentration factor values of 9.4–264 measured in different species of fish suggest bioconcentration in aquatic organisms can be low to high. The principal breakdown product of chlorothalonil in the soil is 4-hydroxy-2,5,6-trichloroisophthalonitrile, which is slightly toxic to aquatic organisms and moderately toxic to birds and mammals. Soil moisture and temperature both promote degradation of chlorothalonil. It has a high degree of binding and low mobility in silty loam and silty clay loam soils, while having a relatively low binding and moderate mobility in sand. Residues of chlorothalonil remain on above-ground crops at harvest but are prone to dissipate overtime. It is a relatively persistent fungicide on plants, based on its rate of application. Chlorothalonil is almost insoluble in water and does not evaporate easily.
Animal Toxicity
Forestomach and the renal proximal tubule are the primary target tissues of chlorothalonil toxicity in Sprague–Dawley rats. Toxicity is characterized by hypertrophy, hyperplasia, vacuolization, and degeneration of renal tubular epithelium and acanthosis, hyperkeratosis, and hyperplasia of the squamous epithelium of the forestomach. Chlorothalonil is a well-known skin and eye irritant. Sustained contact with the squamous epithelium of the forestomach can lead to an inflammatory response. The earliest observation following chlorothalonil administration at 175 mg kg-1 day-1 to rats for varying periods of time for up to 91 days has been characterized by multifocal ulceration and erosion of the mucosa, subsequently progressing to hyperplasia and hyperkeratosis. These lesions have been observed in subchronic and chronic studies in rats and mice (no observed effect level (NOEL) ∼2 mg kg-1 day-1) and in chronic studies appear to be closely related with incidence of neoplasia (NOEL ∼4–21 mg kg-1 day-1). Undiluted chlorothalonil is a strong irritant and produces irreversible corneal, iridal, and conjunctival effects in rabbits. Weakness and sedation precede death in animals given acute toxic doses intraperitoneally. Chronic oral administration to rats results in ataxia. Hematuria, vaginal bleeding, and epistaxis are seen in rats following chronic oral exposure. In chronic dermal exposures to chlorothalonil dissolved in acetone, the no-effect level for irritation is 0.001%. The 0.01% concentration is a mild irritant and 0.1% a moderate irritant. Prolonged exposure of rodents to chlorothalonil results in nephrotoxicity and renal tubular hyperplasia, and these effects, if sustained, can lead to a tumorigenic response. Chlorothalonil produced a dose-related increased incidence of renal tubular adenomas and adenocarcinomas in rats. The oral LD50 in rats is greater than 10 g kg-1. Chlorothalonil is predicted to be a rodent carcinogen via a nongenotoxic mechanism.
Detection in Minnesota Waters
The Minnesota Department of Agriculture (MDA) monitors groundwater and surface water throughout Minnesota for over 180 pesticide-related chemicals. Since the MDA began monitoring for chlorothalonil in 2001, it has only been detected a few times in groundwater and surface water, and all detections have been well below the Minnesota Department of Health (MDH) health-based guidance value (health risk limit: 1 µg/L).[3]
In 2020, the MDA began monitoring for 4-hydroxychlorothalonil, a major degradate of chlorothalonil. From 2020 through 2023, 4-hydroxychlorothalonil has been detected in approximately 10% of groundwater samples (n=888), and all detections have been in the central sands region of Minnesota. 4-Hydroxychlorothalonil has been detected at concentrations above the MDH health-based guidance value (risk assessment advice: 2 µg/L) in groundwater. In 2023, there were 12 detections of 4-hydroxychlorothalonil above the health-based guidance value in groundwater, with a maximum concentration of 12.7 µg/L.
References
[1] Andre et al., V. Andre, P. Lebailly, D. Pottier, et al.Urine mutagenicity of farmers occupationally during a 1-day use of chlorothalonil and insecticides Int. Arch. Occup. Environ. Health, 76 (1) (2003), pp. 55-62
[2] Bellas, J. Bellas, Prediction and assessment of mixture toxicity of compounds in antifouling paints using the sea-urchin embryo-larval bioassay, Aquat. Toxicol., 88 (4) (2008), pp. 308-315
[3] de Castro et al., V.L. de Castro, S.H. Chiorato, N.F. Pinto, Biological monitoring of embrio-fetal exposure to methamidophos or chlorothalonil on rat development, Vet. Hum. Toxicol., 42 (6) (2000), pp. 361-365
You may like
Related articles And Qustion
See also
Lastest Price from Chlorothalonil manufacturers

US $0.00/box2025-09-28
- CAS:
- 1897-45-6
- Min. Order:
- 1box
- Purity:
- 99%
- Supply Ability:
- 20KL per month

US $30.00-10.00/KG2025-04-15
- CAS:
- 1897-45-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg