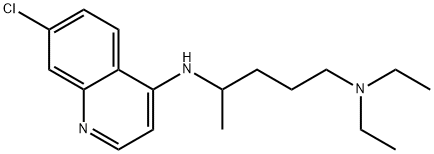
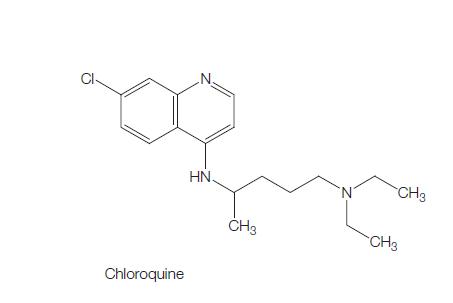
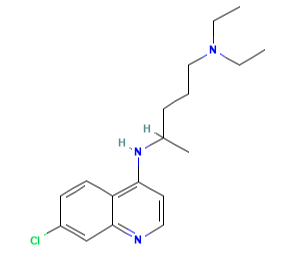
Chloroquine
General description
Figure 1 the molecular formula of Chloroquine
Application and pharmacokinetics
It is used for the treatment of malaria, hepatic amoebiasis, lupus erythematosus, light-sensitive skin eruptions, and rheumatoid arthritis. It has a role as an antimalarial, an antirheumatic drug, a dermatologic drug, an autophagy inhibitor and an anticoronaviral agent. It is an aminoquinoline, a secondary amino compound, a tertiary amino compound and an organochlorine compound. It is a conjugate base of a chloroquine(2+). Chloroquine is an aminoquinolone derivative first developed in the 1940s for the treatment of malaria. It was the drug of choice to treat malaria until the development of newer antimalarials such as [pyrimethamine], [artemisinin], and [mefloquine]. Chloroquine and its derivative [hydroxychloroquine] have since been repurposed for the treatment of a number of other conditions including HIV, systemic lupus erythematosus, and rheumatoid arthritis. [1]
The FDA emergency use authorization for [hydroxychloroquine] and chloroquine in the treatment of COVID-19 was revoked on 15 June 2020. This product and other 4-aminoquinoline antimalarial drugs (such as piperquine, acetaminophen, etc.) mainly act on the red stage of Plasmodium, which may interfere with the replication and transcription of Plasmodium schizont DNA or Hinders its endocytosis, so that the worms die due to lack of amino acids. Chloroquine was granted FDA Approval on 31 October 1949.the option of using chloroquine in the treatment of SARS-CoV-2 should be examined with attention in light of the recent promising announcements, but also of the potential detrimental effect of the drug observed in previous attempts to treat acute viral diseases.
Chloroquine has also been tested in chronic viral diseases. Its use in the treatment of HIV-infected patients has been considered inconclusive and the drug has not been included in the panel recommended for HIV treatment. The only modest effect of chloroquine in the therapy of human virus infection was found for chronic hepatitis C: an increase of the early virological response to pegylated interferon plus ribavirinand, in a small sample size pilot trial in non-responder HCV patients, a transient viral load reductionwere observed.
The possible benefit o fchloroquine, a broadly used antimalarial drug, in the treatment of patients infected by the novel emerged coronavirus (SARS-CoV-2). Based on a demonstrated in vitro effect on SARS-CoV-2 and its known safety profile, both chloroquine and hydroxychloroquine (CQ/HCQ) are currently being used off label to treat COVID-19.
Synthesis
The synthetic process of hydroxychloroquine sulfate(1) was improved. 4,7-Dichloroquinoline(3) and 5-(N-ethyl-N-2-hydroxyethylamino)-2-pentylamine(4) were refluxed in n-butanol with potassium carbonate as the acid binding agent to give hydroxychloroquine(2). Sulfuric acid was directly added dropwise to the n-butanol solution of 2 to obtain 1 with a purity of 99.06% after recrystallization with EtOH. The improved process involved fewer steps and was easy to operate. Using n-butanol as a solvent would effectively avoid the loss of 3 due to sublimation, and using potassium carbonate as an acid-binding agent could decrease the formation of impurities 4-[2-(5-hydroxyethylamino)-pentyl]amino-7-chloroquinoline(5) and N-[2-[(7-chloroquinoline-4-yl)oxy]ethyl]-N-ethylpentane-1,4-diamine(6). The total yield increased from 39% to 69%(based on 3)[2]
Figure2 Synthesis of hydroxychloroquine sulfate
The synthesis process of 1 has been improved, as shown in Figure 3. When preparing 2, use 3 and 4 as raw materials, and use n-butanol instead of toxic phenol as the solvent. 3 Can be fully transformed.In addition, the experiment found that adding a base as an acid binding agent in the reaction significantly reduced impurity 5, while impurity 6 would increase with the increase in the alkalinity of the acid binding agent. Under the condition of a weak base such as potassium carbonate, the content of 6 was less than 1%. When using a medium strong base such as sodium hydroxide, the content of 6 increases to 5%, especially in the presence of a strong base of sodium tert-butoxide, the content of 6 increases to 93%.
Toxicological and Safety
ChloroqChloroquine is an established antimalarial agent that has been recently tested in clinical trials for its anticancer activity. The favorable effect of chloroquine appears to be due to its ability to sensitize cancerous cells to chemotherapy, radiation therapy, and induce apoptosis. The present study investigated the interaction of zinc ions with chloroquine in a human ovarian cancer cell line (A2780). Chloroquine enhanced zinc uptake by A2780 cells in a concentration-dependent manner, as assayed using a fluorescent zinc probe. This enhancement was attenuated by TPEN, a high affinity metal-binding compound, indicating the specificity of the zinc uptake. Furthermore, addition of copper or iron ions had no effect on chloroquine-induced zinc uptake. chloroquine’s anticancer activity.[3,4]uine is an aminoquinoline used for the prevention and therapy of malaria. It is also effective in extraintestinal amebiasis and as an antiinflammatory agent for therapy of rheumatoid arthritis and lupus erythematosus. Chloroquine is not associated with serum enzyme elevations and is an extremely rare cause of clinically apparent acute liver injury.Although the safety of CQ/HCQ is well established in malaria or autoimmune disease, COVID-19 patients could be more vulnerable to side effects because of their advanced age, comorbidities (such as diabetes, obesity and cardiovascular disease), andsubsequent co-medication. Both agents are metabolized via theliver and the kidney. Critically ill patients may have an alteredmetabolism due to changes in hepatic and renal function, which could increase the risk of adverse reactions.
You may like
Related articles And Qustion
Lastest Price from CHLOROQUINE manufacturers

US $10.00-15.00/g2023-03-11
- CAS:
- 54-05-7
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 2546245