Chemical Reactivity of Benzo[c]isoxazole
Benzo[c]isoxazole is a bicyclic-fused heteroaromatic in which the benzene ring is fused with the “c” site of the isoxazole ring with a 10π electron ring system and low stability due to cross-conjugation and disruption of aromaticity. It is also known as anthranil and can be considered as an isomer of the benzoxazoles in which the oxygen atom is located at the 2-position of the ring. A thorough literature survey revealed that a number of these compounds have been used as key intermediates in the synthesis of various drugs such as antimycobacterial, protein kinase inhibitor, and anticancer agents.
Chemical Reactivity
Due to high reactivity, benzo[c]isoxazoles have been used as versatile precursors in organic synthesis. These molecules form 1-alkyl-2,1-benzisoxazolium salts in good yields after the reaction with trialkylorthoformates in the presence of BF3.Et2O and Meerwein’s reagent (Et3OBF4). These quaternary salts react with a variety of nucleophiles to generate a wide range of benzo[c]isoxazolines. Furthermore, these salts have been successfully converted to ortho-alkylaminobenzophenones on treatment with Na2S2O4 in aqueous methanol or zinc in AcOH.
Synthesis
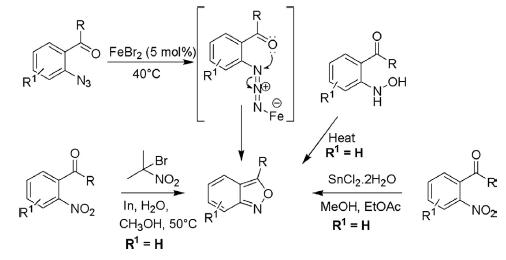
Over the years, a number of synthetic protocols for the synthesis of benzo[c]isoxazoles have been developed by cyclizing the suitably modified ortho-substituted benzenes. These methods include (1) spontaneous cyclization of o-acylphenylhydroxylamines, (2) indium-mediated heterocyclization or reduction in the presence of stannous chloride followed by heterocyclization of 2-nitroacylbenzenes, and (3) Fe(II)-catalyzed intramolecular cyclization of 2-acylarylazides.
![271-58-9 Chemical Reactivity of Benzo[c]isoxazoleBenzo[c]isoxazole](https://www.chemicalbook.com/CAS/GIF/271-58-9.gif)